Sharp and to the Point
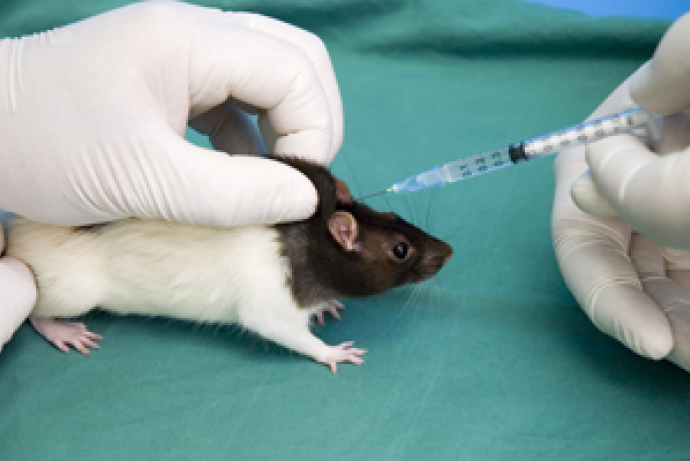
The aim of this Challenge is to develop a device that enables injections in mice without losing material to dead space, allows needles to be changed quickly and safely between animals to ensure sharpness and sterility, and which prevents cross-contamination between animals. This must be competitively priced to facilitate broad uptake across the bioscience sector.
CRACK IT Challenge webinar: A precise, low dead space needle with improved animal welfare
In October 2022, we hosted a webinar to provide an overview of the Precision Injection System developed through the Sharp and to the Point Challenge. Speakers included Dr Mike Irvine and Dr Kate Shenton (Active Needle Technologies Ltd), as well as Challenge Sponsors Ms Sam Izzard (GSK), Dr Lucy Whitfield (University of Sheffield) and Dr Sally Robinson (AstraZeneca). The recording is now available to watch online on the NC3Rs website.
Challenge completed
Through the Sharp and to the point Challenge, the team at Active Needle Technology Ltd have developed a precise injection system that reduces waste by decreasing dead space within syringes, and a needle attachment mechanism to allow for safer exchanging of needles.
Challenge Awarded
Active Needle Technology Ltd have been awarded £99,382 to deliver the project: Ultrasonic, low dead space needle with improved animal welfare. The team is led by Mr Ian Quirk.
Challenge launched
Sponsored by AstraZeneca, GSK, The Royal Veterinary College and The University of Sheffield, this Challenge aims to develop a device that enables injections in mice without losing material to dead space, allows needles to be changed quickly and safely between animals to ensure sharpness and sterility, and which prevents cross-contamination between animals.
Background
Hypodermic needles are used throughout medical research involving animals for injecting substances (e.g. via intravenous, subcutaneous, intraperitoneal or intramuscular routes) or for collecting blood samples. Most needles used to administer material parenterally in animals are designed and sold as single-use disposable needles.
Where needles are re-used, it is commonly done to reduce time and cost because of the involvement of large numbers of mice and/or test material. Two blogs published on the NC3Rs website have focused on the topic of re-using hypodermic needles in day-to-day practice and highlight the associated scientific and welfare concerns 1,2.
The re-use of needles results in a loss of sterility and can increase the risk of infection and disease transmission between individual animals and between cages. Re-use also risks dulling of the needle, potentially increasing the pain and discomfort associated with subsequent injections. These concerns create confounding and unnecessary variables which can impact the quality of scientific data collected.
When needles are re-used in mouse studies, it is most often to address the following:
- Loss of material to dead space. The primary reason for re-use of needles and syringes is loss of valuable material (such as cells, compounds, viruses) in the fluid not expelled due to dead space between the syringe and the needle hub. The materials used in pilot studies are often difficult and expensive to synthesise and are therefore made in small quantities. This, combined with the size of mice and the small volumes administered, mean dead space losses can form a significant percentage of the material needed. An increasing number of studies involve the injection of living cells for example, for xenograft models used in oncology research. To account for dead space losses during administration, an excess of cells is needed. For certain cell lines, these can be difficult to grow and harvest in large quantities without affecting their quality.
- The time needed to change needles between injections. This can be a concern for studies where materials may need to be injected within a limited time into large numbers of animals. For example, changing the needle for each animal in a study where the formulation stability requires all animals to be dosed within an hour of formulation preparation would require additional staff to complete the injections within the necessary timeframe.
It is possible to purchase low dead space needles and syringes. However, they are not available in all needle gauges, and the syringe barrels are often difficult to read for small volumes which can affect the accuracy of small volumes draws. Insulin syringes have been developed that have no dead space. However, these are supplied with the needle attached making them inappropriate for use with substances that cannot be drawn up through the needle (e.g. cells).
All currently available products require significant time to manually change a needle between each animal.
3Rs benefits
There is evidence to show that the single use of a needle can cause significant dulling/deformity of the needle tip 1, and that re-use of these needles may cause transient pain 3,4 and tissue damage at the site of injection. The transfer of material between animals (blood/tissue) can also increase the risk of infection and cross-contamination between cages of mice may occur. Infection and disease transmission can lead to study failure and result in further animal use and welfare concerns as well as loss of time and money. Use of needles more than once can also affect the quality of the scientific data collected as each animal in the cage is treated differently with respect to the process of injection.
In 2017, 1.89 million animals were used in experimental procedures in the UK. Approximately 58% were mice and many of these would have been involved in procedures involving an injection for example, for basic research programmes, administration of materials in efficacy, toxicity and pharmacokinetic studies, or in vaccine batch testing for quality and safety. A small refinement in how substances are delivered would positively impact a high number of animals.
Full Challenge information
Challenge winner
Project team led by:
- Mr Ian Quirk, Active Needle Technology Ltd, £99,382
Assessment information
Challenge Panel membership
| Name | Institution |
|---|---|
| Dr Martino Picardo (Chair) | Independent |
| Dr Sally Robinson (Sponsor) | AstraZeneca |
| Dr Sam Izzard (Sponsor) | GSK |
| Dr Sue Sparrow (Sponsor) | GSK |
| Dr Helen Marriott (Sponsor) | The University of Sheffield |
| Dr Lucy Whitfield (Sponsor) | Royal Veterinary College London |
| Mrs Margaret Parton | Independent |
| Mr James Bussell | University of Oxford |
| Professor Paul Flecknell | Newcastle University |
| Mr Ian Rosewell | The Francis Crick Institute |
| Professor Dionysios Douroumis | University of Greenwich |
Through this Challenge, the team at Active Need Technology Ltd have developed a Precision Injection System to improve preclinical injections of rodents. The system enables high accuracy and precision injection leading to minimal waste of injectate.
Most needles used in animal studies are designed and sold as single-use disposable needles. When needles are re-used, it is often done to reduce the loss of valuable material to dead space or to reduce the time needed to change needles between injections. The unique system addresses these key issues by almost eliminating the dead space within syringes, greatly reducing waste, and allowing rapid and easy needle exchange.
The Precision Injection System also encourages the use of new, sterile needles for each animal and reduces the time to inject large cohorts, therefore minimising any pain that would come from reusing a dulled needle. Refining the delivery of substances by avoiding the reuse of single-use needles also improves animal welfare by reducing the risk of cross-contamination and infection or disease being spread from animal to animal. High accuracy dosing also ensures that the animal receives the specified dose, avoiding unintentional over- or underdosing.
This successful project has been further supported by the BGS award scheme to assist in transition into the commercial sphere.
This Challenge was delivered with the support of the sponsors AstraZeneca, GlaxoSmithKline, and the University of Sheffield. Active Needle Technology Ltd continue to work with the Sponsors to validate and implement the technology for real-world application.
For more information on the Precision Injection System and Active Needle Technology Ltd, please visit their website.
Active Needle Technology Ltd has developed and evaluated a Precision Injection System for use in preclinical injections of rodents. The system is a handheld syringe with a unique design that virtually eliminates dead space (< 2µl per injection) to increase accuracy and minimise the amount of injectate wasted, thereby reducing the volume of valuable injectate that must be formulated/synthesised.
The design allows for convenient single-handed exchange of needles, reducing the time to inject large cohorts and encouraging the use of a new needle for each animal. The injection is controlled by a footswitch permitting focused attention on subject handling and dose administration during the injection process. An ergonomic handpiece allows for different ways of holding according to user preference and dosing route.
View the Precision Injection System in use below:
Procedures in the video are performed on mice.
Features





For more information on the Precision Injection System, please contact Active Needle Technology:
Email Kate Shenton (Scientist and System Advocate)
Visit the Active Needle Technology website