Forming clots in in vitro blood flow
Dr Alan Harper and PhD Student Jacob Ranjbar have developed an artificial human blood vessel to study blood clotting in vitro and replace the use of mice in thrombosis research. They have successfully disseminated the model to the thrombosis community, including for new applications within cardiovascular research.
Platelet biology is currently studied in vivo
Platelets play an important role in coordinating blood clotting at the site of blood vessel injury, ensuring that damaged blood vessels are repaired and excessive blood loss is prevented. However, when platelets become activated inappropriately inside undamaged blood vessels they can block the blood supply to the heart and the brain, triggering heart attacks and strokes. The molecular events underpinning normal and abnormal blood clotting are not currently fully understood. Predominantly these are studied using intravital microscopy where mice are anaesthetised and treated with fluorescent antibodies to label the platelets. Accessible arteries or veins are mechanically or chemically damaged and the resultant blood clot monitored fluorescently by microscopy.
Engineering an artificial human blood vessel
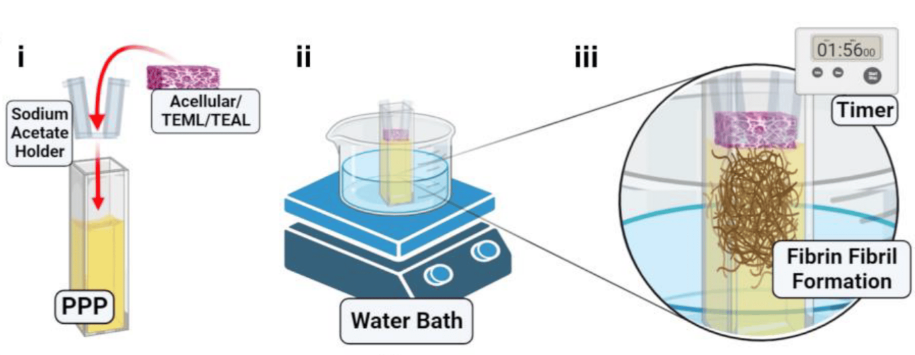
Alan previously used a tissue engineering approach to develop a 3D multi-layered artificial human blood vessel. The model combines human cells with a collagen scaffold to reproduce the tunica media and tunica intima layers of the blood vessel which are essential for blood clotting. Alan was awarded an NC3Rs Studentship in 2018 to further develop the model to study platelet activation in vitro. Jacob, the PhD student, incorporated blood samples from healthy human volunteers into the artificial vessel and demonstrated that these form blood clots when they are mechanically damaged, but not when they are intact – replicating the properties of normal human arteries. He also designed and fabricated a novel 3D printed flow chamber to create blood flow conditions through the artificial vessel which are similar to those found in the human body.
Dissemination to thrombosis research groups
The artificial human blood vessel model has the potential to replace mice within the thrombosis research community, and Alan and Jacob have collaborated extensively to achieve this. They established a knowledge exchange with NC3Rs Studentship holder Dr Alice Pollitt and PhD student Carly Kempster at the University of Reading. In this exchange Alan and Jacob trained Alice and Carly in making and using the artificial blood vessel and in turn they received training in methods of delivering compounds to platelets. These two techniques have complimentary applications for reducing the use of mice in thrombosis research, and Alan and Alice are continuing to work together to merge their models to achieve enhanced 3Rs impact supported by an NC3Rs Project grant.
Further collaborations include with Dr David Cabrera (Keele University) who undertook training in the 3D printing of flow chambers which he has then modified for his work studying the role of magnetic hyperthermia in the treatment of deep vein thrombosis. David began training in the vascular tissue engineering techniques used in the project in 2020 and is now helping to further develop the model to use in the study of venous thrombosis. To further encourage the replacement of mice by thrombosis research groups, Alan and Jacob have published a review paper outlining available 3D tissue engineering approaches to modelling the arterial wall and the benefits and limitations of each of these [1].
Transfer to new cardiovascular research applications
Alan and Jacob are now supporting Dr Prachi Stafford (Sheffield Hallam University) to transfer the model to a new area of cardiovascular research. Prachi plans to use the model to investigate how bacterial species associated with gingivitis could contribute to the onset of acute cardiovascular events. As part of this collaboration Prachi has provided feedback on the adoptability of the model, and Alan will use this to continue to refine the set-up to make the model more accessible to the wider cardiovascular community.
References
- Ranjbar J et al. (2023). Developing human tissue engineered arterial constructs to simulate human in vivo thrombus formation. Platelets 34(1): 2153823. doi: 10.1080/09537104.2022.2153823
- Ranjbar J et al. (2023). Developing Biomimetic Hydrogels of the Arterial Wall as a Prothrombotic Substrate for In Vitro Human Thrombosis Models. Gels 9(6):477. doi: 10.3390/gels9060477

