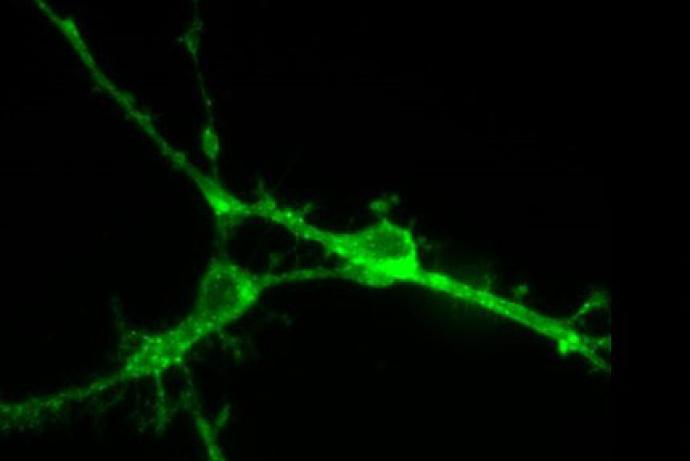
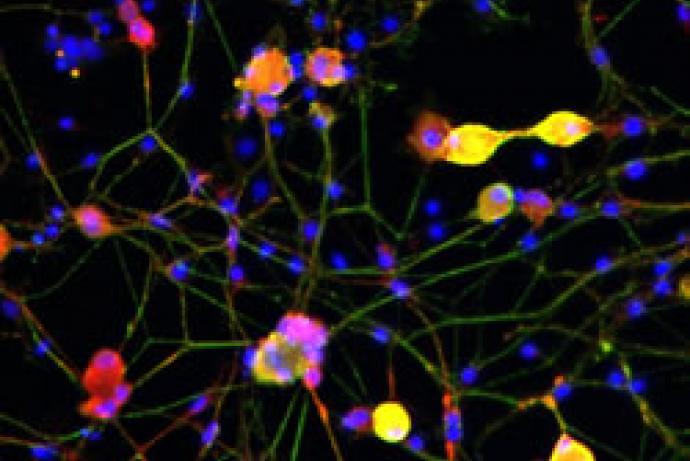
Tetanus vaccine production currently relies on animal models for assessing residual tetanus toxoid toxicity. As an alternative to these models, researchers at the University of Sheffield have engineered mouse immortalised neurons that carry a stabilised VAMP reporter molecule, overcoming the challenges that have previously limited the development of cell-based assays for this purpose. This quantitative cell-based neurotoxin assay would be suitable for both tetanus and botulinum product development and partners are sought to help validate the assay using toxoid samples. This approach has the potential to reduce the thousands of animals used in the production of tetanus and botulinum vaccines annually.
Tetanus neurotoxin is a large multi-subunit protein that poisons spinal cord neurons and blocks neurotransmission in three steps (Rossetto et al., 2013). The heavy chain of the tetanus neurotoxin consists of a binding domain for neuronal ganglioside attachment (step 1) and a translocation domain that mediates penetration of the tetanus protease into the cytosol (step 2) for cleavage of the neuronal protein VAMP (vesicle-associated membrane protein; step 3), thereby causing blockade of neurotransmission (Rossetto et al., 2013). Tetanus toxoids (i.e. inactivated preparations of tetanus neurotoxin) are used for the production of tetanus vaccines. To exclude the risk of residual toxicity, each batch of tetanus toxoid undergoes strict safety assessment, by in vivo animal testing (usually mice and guinea pigs). This method induces widespread physiological changes that do not detect the primary cause of tetanus toxicity – the cleavage of VAMP molecules in neurons. These studies also have significant welfare concerns, and carry the highest welfare severity level under the UK licensing system.
For several decades there has been a strong desire to replace these slow, costly, unethical animal assays with a cell-based assay that enables sensitive evaluation of all key steps in tetanus action. To date, two major problems have hindered the development of replacement cell-based assays for testing residual tetanus activity:
- The majority of cell lines do not produce neuronal gangliosides required for tetanus binding and thus lack the sensitivity necessary to be used in an assay. The use of primary neurons or embryonic cell-derived neurons pose their own challenges as they have to be freshly derived from tissue or require complicated protocols and weeks/months to become fully differentiated.
- The absence of VAMP protein or the rapid degradation of tetanus-cleaved VAMP substrate, which precludes detection of neurotoxin activity.
A suitable method capable of detecting low concentrations of active tetanus neurotoxin in mixtures containing large amounts of inactivated toxoid molecules is required.
Reference
- Rossetto O, Scrozeto M, Megighain A, et al. (2013). Tetanus neurotoxin. Toxicon 66: 59-63. doi:10.1016/j.toxicon.2012.12.027.
Researchers at the University of Sheffield have engineered a VAMP-reporter incorporated into an immortalised neuronal cell line which overcomes the current issues with cell-based assays, and will accelerate vaccine production and testing. The tetanus-cleaved stabilised VAMP can be detected using an enzymatic reporter with the highest possible sensitivity within 24 hours following toxoid application. The method detects active tetanus neurotoxin molecules based on tetanus’ three characteristics: specific receptor-binding, translocation of tetanus protease, and its proteolytic activity. By taking into account all relevant functional characteristics, this assay fully discriminates between toxic and detoxified molecules, in contrast to other in vitro assays that solely rely on one or two toxin functions (e.g. endopeptidase assays) (Fernández-Salas et al., 2012). This novel assay can ensure batch-to-batch consistency during tetanus toxoid production.
The cell-based VAMP reporter assay can also be used in the manufacture of botulinum products whether for vaccine development or for use in biomedicine. The researchers have specifically verified activity of botulinum neurotoxin type B and D using this cell-based assay. This novel user-friendly clonal cell line that differentiates within two days could prove useful as a general-purpose “one size fits all” method for evaluation of tetanus and all botulinum neurotoxins since this cell line will have both VAMP and SNAP25 neurotoxin targets.
This assay has the potential to underpin testing of tetanus products throughout the world. Tetanus vaccine is on the WHO list of essential global medicines and thus potential market size for this cell-based assay is significant. In 2012, the WHO launched their Global Vaccine Action Plan, which aims to bring global immunisation coverage for tetanus by 2020. Major vaccine producers such as GlaxoSmithKline, Sanofi Pasteur and Mass Biologics are committing resources to provide cheaper and larger stocks of common vaccines to developing countries.
Reference
- Fernández-Salas E, Wang J, Molina Y, et al. (2012). Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS One 7(11): e49516. doi:10.1371/journal.pone.0049516.
Partners are sought to help further develop, validate and commercialise this cell-based VAMP-reporter assay for tetanus toxoid testing. The concept has been validated using polyclonal antibodies so, ideally, the partner would assist to incorporate highly specific monoclonal antibodies, aptamers or affibodies that recognise the tetanus-cleaved end of the VAMP polypeptide, making the assay even more standardised. The partner would assist in selecting the most sensitive clonal cell lines using real toxoid samples. The assay can also be used for detection of botulinum neurotoxin activities, and therefore partners with expertise in botulinum product development are also sought.
Information about IP
A review of the scientific literature indicates the project is novel and The University of Sheffield is considering IP protection.
Tens of thousands of rodents are used globally each year in the production of tetanus vaccines for (i) production of tetanus neurotoxin, (ii) its chemical inactivation, (iii) testing residual tetanus neurotoxin activity and (iv) testing toxoid immunogenicity. The first three stages require analysis of the lethal effects associated with neuromuscular paralysis which are normally assigned the highest severity limits by regulatory authorities. Each batch of tetanus vaccine produced contains 100,000 human doses. Assuming that 100 million immunisations take place every year in the world, the industry needs to validate 1,000 batches of the tetanus vaccine. If, using conservative estimates, 30 animals are used in a single vaccine batch validation, one can assume that industry uses 30,000 animals for testing every year. Since roughly an equal number of immunisations take place in veterinary medicine, at least 60,000 guinea pigs and mice are used in tetanus vaccine production each year. Furthermore, evaluation of “specific toxicity of the tetanus component” in guinea pigs is required for all combination vaccines further increasing animal use. The proposed development and application of this cell-based neurotoxin assay has the potential to replace many of these animal tests.