Researchers at Manchester Metropolitan University (MMU) offer a new biosensing platform utilising plastic antibodies that is compatible with both electrochemical detection and a novel thermal technique. The sensor platform can be tailored to any application: by altering the plastic antibody, different biological targets (from small molecules to cells) can be measured. These “plastic” antibodies that are low-cost, very stable, can be manufactured in bulk quantities and reduce the number of animals required in comparison to traditional antibodies. Academic and industrial collaborators, with access to biological samples or with engineering/ software expertise, are sought to further validate and develop this approach to ensure maximum uptake of the technology.
Through CRACK IT Solutions Dr Peeters partnered with MIP Diagnostics and a clinical partner. With the Solutions funding awarded the consortium prepared and validated a high affinity biosensor using molecularly imprinted polymer (MIP) nanoparticles for the thermal detection of cardiac biomarkers. For every MIP that is produced for a specific target, this technology avoids the immunisation and subsequent use of these animals.
There is a growing market to develop tests for the straightforward, fast and low-cost detection of biomolecules. The recognition element in these widely-used tests is traditionally based on antibodies. A 2015 report stated that the market for antibodies in the United States was up to $2.5 billion per year and growing (Baker, 2015). Besides ethical concerns, as antibodies are obtained from animals, they are expensive and unstable, which influences reliability (Kusnezow and Hoheisel, 2002). The loss in time and resources from poorly characterised antibodies is estimated at $800 million per year, which does not take into account the waste of samples and false conclusions from experiments (Baker, 2015).
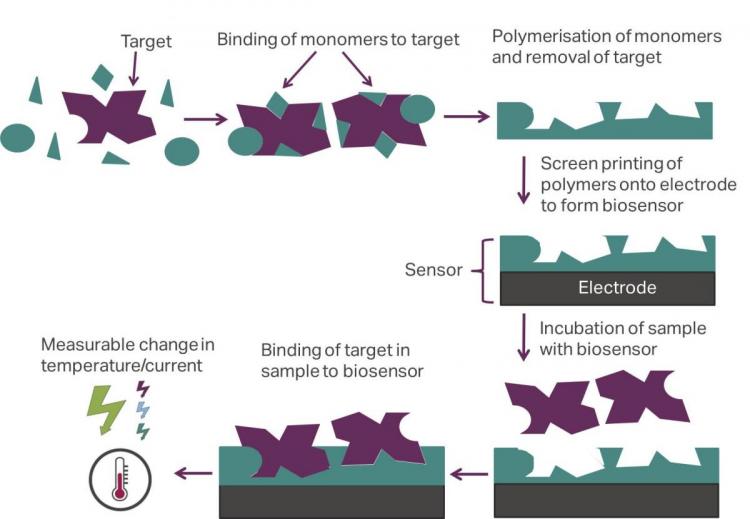
Polymers, or “plastic” antibodies, can mimic the properties of natural antibodies and are able to overcome these drawbacks. These synthetic antibodies, referred to as molecularly imprinted polymers (MIPs), are made by polymerizing vinylic monomers in the presence of a specific target (Haupt and Mosbach, 2000). After removal of the target, a porous structure is obtained with imprints that have a high specificity towards the target. MIPs are an emerging technology that are finding their first commercial applications; chromatography columns packed with MIP particles used for extraction can be obtained from companies such as Biotage and Sigma Aldrich (Nestora et al., 2016).
Advantages of MIPs over antibodies include superior stability; MIPs can operate up to 100˚C and are stable in organic solvents and extremes of pH. In addition, they are prepared in bulk quantities and give consistent results, contrary to batch-to-batch variability observed in the case of antibodies. MIPs are polymers, which makes them fundamentally different to other high affinity synthetic receptors, including affimers (protein complexes) or aptamers (oligonucleotide or peptide-based). Molecular imprinting technology is versatile and it is possible to tailor the polymer for specific targets; meaning it is possible to detect a wide range of biological targets from small ions to larger proteins, such as biomolecules in this instance.
References:
- Baker M (2015). Antibody anarchy: A call to order. Nature 527(7579): 545-51. doi:10.1038/527545a.
- Haupt K, Mosbach K (2000). Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 100(7): 2495-2504. doi:10.1021/cr990099w
- Kusnezow H and Hoheisel JD (2002). Antibody microarrays: promises and problems. Biotechniques 33: suppl 14-23.
- Nestora S, Merlier F, Beyazit S, et al. (2016). Plastic Antibodies for Cosmetics: Molecularly Imprinted Polymers Scavenge Precursors of Malodors. Angew. Chem. Int. Ed. 55(21): 6252-6256. doi:10.1002/anie.201602076.
Manchester Metropolitan University offer a new biosensing platform utilising plastic antibodies that is compatible with both electrochemical detection and a novel thermal technique. The MIPs are integrated into Screen-Printed Electrodes (SPEs) by mixing the imprinted polymer particles with screen-printing ink (Peeters et al., 2016) and transferring it onto a substrate. Printing can be done on a variety of polymer and metallic substrates and allows fine tuning of the sensor to the required application. This process is one of the most promising approaches for production of biosensors as it ensures rapid, accurate and low-cost (disposable sensors) in situ analysis and is easily integrated into portable devices.

The SPEs, functionalised with the plastic antibodies, are incubated with the analyte and binding of targets to the polymer layer is reflected in temperature changes. The temperature changes are measured with a set up developed at MMU that analyses the transport of heat-flow through the samples, and this is used to determine the concentration of the analyte. This can be done with two different thermal methods, which are both patented and referred to as the Heat-Transfer Method (HTM) and Thermal Wave Transport Analysis (TWTA) (van Grinsven et al., 2014; van Grinsven et al., 2016). The developed sensors are versatile and can be measured with other read-out strategies, such as optical methods (change in colour), gravimetry (extremely sensitive mass balance) and electrochemical techniques such as chronoamperometry (van Grinsven et al., 2014; van Grinsven et al., 2016). This thermal device, which costs only £1000, is portable and can be brought on-site to perform measurements in real-time. This is read-out technology is advantageous in comparison to existing methods (e.g. microcalorimetry) that require sophisticated equipment not standardly available in labs. The thermal detection is performed by automated software, is easy to use, and measurements can be directly transferred to other locations.
The unique advantage of using polymer antibodies is that they can be tailored for multiple applications from detecting neurotransmitters to bacteria. The low cost (£1 per sample) of the disposable polymer-functionalised electrodes and ability to measure samples on-site, it is extremely suitable for screening purposes in remote geographical areas with limited resources. The measurement time of the biosensor platform is approximately 2 min/sample (van Grinsven et al., 2014), which is faster than traditional ELISA antibody assays. In addition, there is the possibility to detect a wide variety of compounds in array format; such as determining various contaminants (heavy metal, pollutants, organic dyes) in wastewater.
References:
- Peeters M, van Grinsven B, Foster CW, et al. (2016). Introducing Thermal Wave Transport Analysis (TWTA): A Thermal Technique for Dopamine Detection by Screen-Printed Electrodes Functionalized with Molecularly Imprinted Polymer (MIP) Particles. Molecules 21(5): 552-564. doi:10.3390/molecules21050552.
- van Grinsven B, Eersels K, Peeters M, et al. (2014). The Heat-Transfer Method: A Versatile Low-Cost, Label-Free, Fast, and User-Friendly Readout Platform for Biosensor Applications. ACS AMI Mater. Interf. 6(16): 13309-13318. doi:10.1021/am503667s.
- van Grinsven B, Eersels K, Akkermans O, et al. (2016). Label-Free Detection of Escherichia coli Based on Thermal Transport through Surface Imprinted Polymers. ACS Sens 1(9): 1140-1147. doi:10.1021/acssensors.6b00435.
This project is a unique collaboration between chemists, biophysicists and engineers. Researchers at MMU now seek additional academic partners, as well as industry partners, to further validate and develop this approach to ensure maximum uptake of the technology.
MMU researchers are seeking partners from academia and industry for:
- Access to biological samples. The researchers are particularly interested in blood or serum samples from patients with bacterial infections or irregularities in neurotransmitter levels.
- Engineering/software expertise. For this the researchers are interested in additional support in the miniaturisation of the set up and development of an app that improves usability and can work alongside the sensor to transform the raw data into a value, such as a number (as with the glucose sensor) or a colour.
- Expertise in and/or equipment for high-throughput screening.
Users to implement the methodology to replace existing immunoassay tests. For this MMU researchers offer both a licence to use the system in house or a contract service where measurement of samples is carried out within their lab.
Antibody generation for research and development currently requires the immunisation and use of between one and five animals (including mice, rabbits, goats and primates) for each batch. For every MIP that is produced for a specific target, this technology avoids the immunisation and subsequent use of these animals. In 2013, nearly 10,000 animals were used in the UK for the production of monoclonal and polyclonal antibodies (Home Office Statistics of Scientific Procedures on Living Animals, 2013). In the Netherlands, this number was greater than 25,000 – meaning there is high potential across Europe for a large reduction in animal use for antibody production. Since this polymer technology is extremely versatile and can be applied to a wide range of biomolecules, there is the possibility to directly replace the use of traditional antibodies for use in bioassays in the area of diagnostics, clinical applications (healthcare), and also throughout the food, chemical and pharmaceutical industries.
References:
- Home Office Statistics of Scientific Procedures on Living Animals, Great Britain, report 2013. Available at www.gov.uk/government/statistics/statistics-of-scientific-procedures-on-living-animals-great-britain-2013.
Overview | Impact | Publication
Overview
With the support of CRACK IT Solutions funding, Dr Peeters is now working with MIP Diagnostics Limited and a clinical partner, to prepare and validate a biosensor using molecularly imprinted polymer (MIP) nanoparticles for the thermal detection of cardiac biomarkers. The biosensor will be validated using cardiac biomarkers in human serum samples from patients diagnosed with myocardial infarction, but is applicable for use in other industries such as basic research or pharma (e.g. detection of cardiac biomarkers in animals). After consulting with end-users of the technology, a commercial prototype of the portable biosensor platform will be developed and showcased to demonstrate the technology and identify potential commercialisation partners.
Impact
This project developed highly selective, high affinity molecularly imprinted polymer nanoparticles (nanoMIPs) for cardiac biomarker ST2 and heart-type fatty acid-binding protein (h-FABP). NanoMIPs to ST2 and h-FABP had KD values (a measure of affinity) of 4 and 14 nM respectively, figures well below that considered to be high affinity (100 nM). These were subsequently functionalised via dip coating onto thermocouples to develop a novel polymer-based biosensing platform.
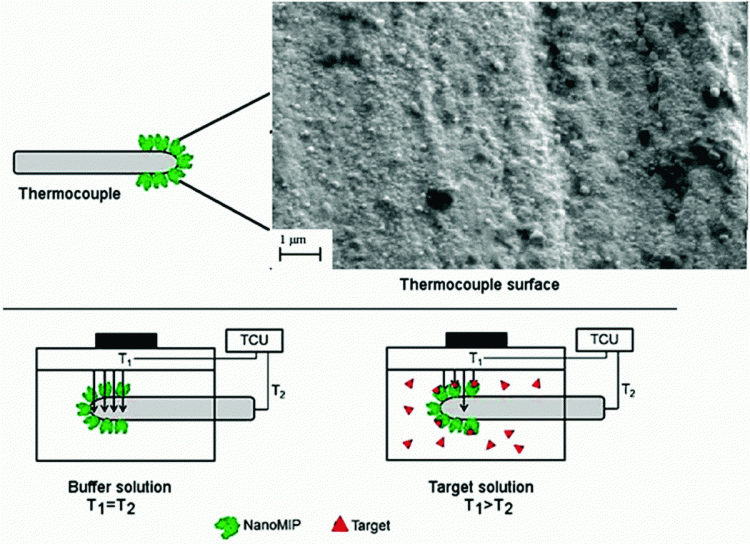
The functionalised thermocouples were inserted into flow cells connected to a thermal device (Figure 1). The thermal resistance, which is defined as the temperature gradient divided by the power to maintain the heat sink at a fixed temperature, was monitored after the addition of biomarker solutions (Canfarotta et al., 2018). Upon binding of the biomarker to the nanoMIP layer, the temperature recorded by the thermocouple (T2) drops, which leads to an increase in the overall thermal resistance.
Figure 1. Thermocouples are functionalised with nanoMIPs and inserted into a flow cell of which T1 is controlled by a temperature controlled unit (TCU). Binding of the biomarker to the nanoMIP layer increases the resistance at the solid-liquid interface, and thereby decreases the temperature measured by the thermocouple (T2).
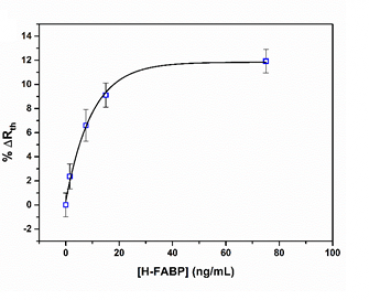
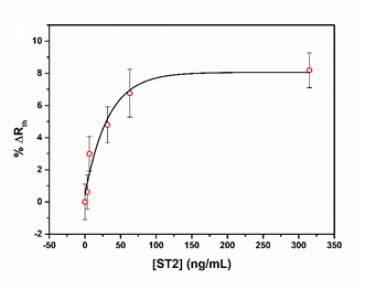
Sequential injections of increasing concentration of h-FABP or ST2 (0.1 – 5 nM) resulted in an increase in the measured thermal resistance (Rth). The measurements were performed in triplicate (three independent measurements and for each measurement a novel functionalised thermocouple was prepared) in order to ensure reproducibility of the sensor platform. The data can be fitted with a standard dose-response curve (R2= 0.99) with limits of detection of ~15 ng/mL for ST2 and 4 ng/mL for h-FABP. The sensitivity of these sensors is therefore similar to traditional immunoassays for these biomarkers, which typically have limits of detection in the order of 1-5 ng/mL (Wodzig et al., 1997; Dieplinger et al., 2015).
Figure 2. The relative change in thermal resistance upon exposure of the nanoMIPs for h-FABP (top) and ST2 (bottom) to its original biomarker concentration. No significant change in thermal resistance was observed for exposure of the functionalised thermocouples to similar biomarkers.
The limit of detection of these sensors is in the physiologically relevant range, and therefore we can pick up elevations of these biomarkers after cardiac injury (Willemsen et al., 2015; Sritara et al., 2015). The measurement can be taken within the required five minutes after calibration is performed. Ethical approval is being sought to use relevant patient samples to validate the nanoMIP functionalised thermocouples. Further work is planned to validate the thermocouple against electrochemical methods.
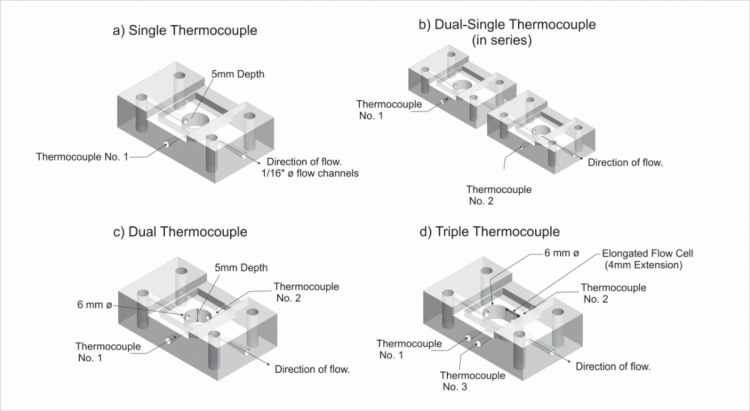
The flow cell was redesigned to enable three or four thermocouples to be inserted into the same flow cell, which multiplexes the system and allows for automatic calibration. All different designs are schematically shown in Figure 3.
Figure 3. Schematic design of the flow cell with a single thermocouple (a), to various flow cells in series (b) and multiplex formats (c,d).
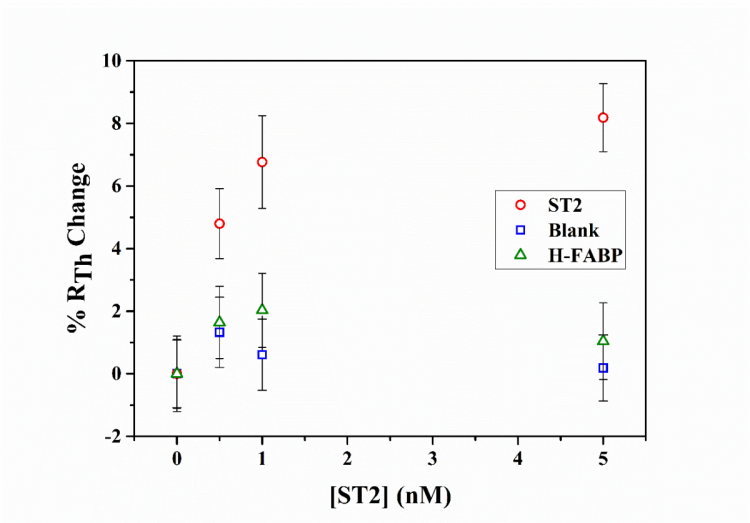
The various flow cell designs were tested against each other by comparing their response upon exposure to 1-5 ng/mL ST2 concentrations (n=3). It was observed that the triple thermocouple design had the lowest limit of detection, and it was possible to measure ST2 concentrations of 10 ng/mL in spiked serum samples without any sample pre-treatment. Mixtures of cardiac biomarkers can be tested, an example is shown in Figure 4 where the response of the triple thermocouple design (d) is shown with one ST2 functionalised thermocouple, one h-FABP functionalised thermocouple, and one blank thermocouple.
Figure 4. Relative change in thermal resistance when thermocouples non-functionalised, functionalised with ST2, and functionalised with h-FABP nanoMIPs were exposed to serum samples spiked with ST2.
The nanoMIP functionalised thermocouples have a high affinity and selectivity, have limits of detection similar to traditional antibody-based assays and can enable the level of multiple biomarkers to be assessed simultaneously. This platform is also low-cost, easy to produce, and has potential for re-usability when compared to traditional ELISA assays. Work to continue the development of the technology towards commercialisation is ongoing.
One to five animals are used to produce the antibodies for one ELISA assay, but these antibodies can be replaced with 0.2 mg of nanoMIPs per 96 well plate. During the project, the team prepared 10 mg of nanoMIPs for ST2 and h-FABP, which is sufficient to replace 50 ELISA assays and replace the use of 50 to 250 animals, however many more nanoMIPs can be prepared. Researchers at the faculty of Healthcare at Manchester Metropolitan University have performed around 50 tests using these nanoMIPs, with an expected further 150 tests as part of a three year project. This would normally equate to the purchase of two ELISA kits, but instead, two to ten animals are replaced using the nanoMIP technology. Dr Peeters will continue to use ST2 and h-FABP target nanoMIPs in her research group at Newcastle University and introduce the technology to collaborators at Maastricht University and Université Libre de Bruxelles.
Throughout this project Dr Peeters has been closely collaborating with MIP Diagnostics and through their website the nanoMIPs developed in this project can be ordered, as well as several other available targets. Further work is planned together to expand the sensor to an assay format that will include other cardiac biomarkers, which we expect will significantly enhance the reliability of the test. Based on the success of the project, a Knowledge Transfer Partnership has been secured with industrial partner Cambridge Medical Technologies. This Solution has increased collaborations with industry and Dr Peeters is in contact with companies interested in adapting the technology to other proteins in the area of food safety and water quality. Further information about the technology can be found here.
Publication
Crapnell R, Canfarotta F, Czulak J, et al. (2019). Thermal Detection of Cardiac Biomarkers Heart-Fatty Acid Binding Protein and ST2 Using a Molecularly Imprinted Nanoparticle-Based Multiplex Sensor Platform. ACS sensors doi: 10.1021/acssensors.9b01666
References
Canfarotta F, Czulak J, Betlem K, et al. (2018). A novel thermal detection method based on molecularly imprinted nanoparticles as recognition elements. Nanoscale 10(4): 2081-2089. doi: 10.1039/c7nr07785h
Wodzig KWH, Pelsers MMAL, van der Vusse GJ, et al. (1997). One-Step Enzyme-Linked Immunosorbent Assay (ELISA) for Plasma Fatty Acid-Binding Protein. Annals of Clin. Biochem 34(3): 263-268. doi: doi.org/10.1177/000456329703400307
Dieplinger B, Mueller T, (2015). Soluble ST2 in heart failure. Clin. Chim. Acta 443: 57-70. doi: 10.1016/j.cca.2014.09.021
Willemsen RTA, van Severen E, Vandervoort PM, et al. (2015). Heart-type fatty acid binding protein (H-FABP) in patients in an emergency department setting, suspected of acute coronary syndrome: Optimal cut-off point, diagnostic value and future opportunities in primary care. European J. General Practice 21(3):156-163. doi: 10.3109/13814788.2015.1013934
Sritara JEP, deFilippi CR, Wang TJ. (2015). Soluble ST2 testing in the general population. Am. J. Cardiol., 115 (7 Suppl), 22B–5B. doi: 10.1016/j.amjcard.2015.01.036