Inovotion has developed a new chick egg chorioallantoid membrane (CAM) assay to reduce animal use and attrition rates in anticancer drug development. The new approach requires only minute amounts of drug and combines efficacy and toxicity studies making the drug development process faster, more reliable and less expensive. Inovotion are now seeking partners from industry or academia able to provide a diversity of novel reagents or compounds with preclinical/clinical data available to test in the model.
Through CRACK IT Solutions Inovotion partnered with AstraZeneca. With the Solutions funding awarded the consortium assessed the toxicity and efficacy of a novel class of antibody drug conjugates using a pyrrolobenzodiazepines (PBD) warhead. The ranking from the most to the least efficacious using the chicken egg model was the same as that observed in the mouse model (using historic data) demonstrating good correlation. This model could replace mouse studies for the pre-screening of ADCs, in terms of in vivo efficacy and toxicity.
This project and its impacts are featured as a case study in the 2019 CRACK IT Review.
Attrition is a major issue in anticancer drug development. Only 5% of agents that have anticancer activity in preclinical development are licensed after Phase III testing, making the drug development process enormously costly and inefficient (Kola and Landis, 2004). Poorly predictive preclinical oncology models are a major factor in this high failure rate. Current preclinical efficacy and toxicity testing strategies rely heavily on animals, specifically rodent xenograft models in which human cancer cells are implanted intraperitoneally in mice and left to grow into tumours. These are then treated with candidate drugs for several weeks or more to assess the drugs ability to inhibit tumour growth or invasion/metastasis. These models do not represent the primary tumours from which they are derived in terms of heterogeneity and drug resistance, leading to substantial limitations which affect their utility and validity in anticancer drug development (Ocana et al., 2010). These models also use considerable numbers of animals. The development of more predictive preclinical models will be critical to prevent further anticancer drug failures.
References
- Kola I and Landis J, (2004). Can the pharmaceutical industry reduce attrition rates?. Nat Rev Drug Disc 3: 711-5. doi:10.1038/nrd1470.
- Ocana A, Paniella A, Siu LL, et al. (2010). Preclinical development of molecular-targeted agents for cancer. Nat Rev Clin Oncol 8 (4): 200-9. doi:10.1038/nrclinonc.2010.194.
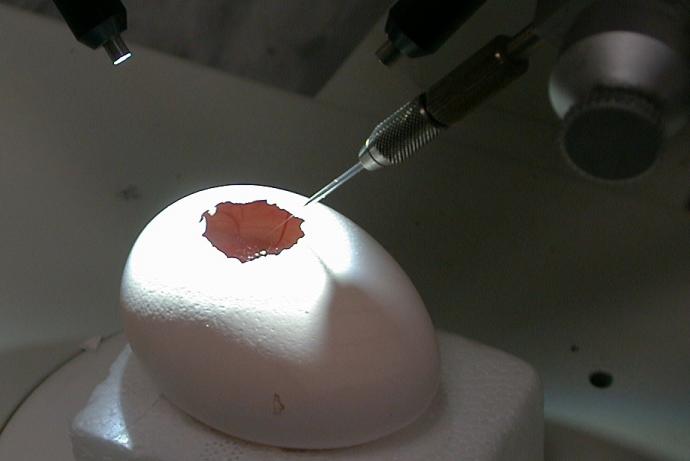
Inovotion’s model uses tumour cells deposited as a homogeneous mass on the chorioallantoid membrane (CAM) of embryonated eggs. This allows tumour growth, invasion into the vasculature and possibly the embryo, and toxicity to be measured simultaneously in controlled conditions and in response to potential anticancer drugs. Measuring efficacy and toxicity of anticancer drug candidates in the same model is beneficial from a scientific perspective and may prove an attractive proposition for company internal decision making processes, reducing the number of compounds entering regulatory in vivo toxicology studies and subsequent animal use.
The initial assays are focused on improving lead optimisation of anticancer compounds; measuring quantitatively and simultaneously the impact on tumour growth and metastatic invasion as well as the toxicity of lead candidates. Inovotion’s test results are equivalent to the current ‘gold standard’ mouse xenograft models but are much faster, more reliable, less expensive and need only minute amounts of drug (>1,000 times less than in mouse models). This demonstrates the potential of the in ovo model to significantly reduce animal use. Inovotion’s proprietary tests are applicable to any preclinical anticancer drug discovery program and could be used more broadly for the early evaluation of the toxicity of any new drug candidate. With further development, Inovotion hope to demonstrate how their model can also improve anticancer drug development beyond current mouse models. By extension, the model could also be useful to test the toxicity of a broad range of chemicals, including industrial or cosmetic.
Inovotion have developed tests based on twelve human tumour cell lines, with a limited number of commercial drugs. To complete these validation studies and demonstrate the utility of the approach over current mouse models, Inovotion would like to measure the activity and toxicity of a larger set of anticancer drugs, whether marketed or in development, and compare the results to those obtained with the mouse tests and available clinical data. Collaborators within industry or academia are sought to provide a diversity of novel reagents or compounds (i.e. small molecules, antibodies, nucleic acids) with preclinical/clinical data available to test in the model. Inovotion and their partner would jointly publish the results of this further development/validation work, whilst keeping the nature of the molecules confidential. Inovotion would also welcome input from their partners to improve the model according to the collaborators needs.
Many millions of mice are used annually in cancer drug development. In the UK, just under 500,000 mice were used in non-toxicology cancer research in 2012. Although the Inovotion tests will not completely replace the use of mouse xenograft models in anticancer drug development, INOVOTION estimate from their initial validation work that they have the potential to reduce the above numbers by 20-30%, and perhaps more. As an example, many current customers are choosing to use only the Inovotion assay for lead optimisation once they have observed the power of the approach, replacing up to 50 mice that would typically be used per compound, dependent on the number of rounds of lead optimisation.
Overview
With support of funding from the Solutions scheme, Inovotion will work with a large pharmaceutical company (Top ten) to assess the toxicity and efficacy of a novel class of antibody drug conjugates using a pyrrolobenzodiazepines (PBD) warhead. Data from the CAM model will be compared to existing in vivo mouse data provided by the pharmaceutical company to evaluate the effectiveness of the model as a cost-effective compound screening method.
If successful, Inovotion’s novel technology could dramatically reduce the use of rodents in cancer drug discovery, as more candidate molecules will be filtered out before progressing to testing in classical in vivo models.
Impact
The purpose of this study was to validate Inovotion’s chicken egg model as a tool to assess the efficacy and toxicity of novel therapeutic antibody-drug conjugates (ADCs) for oncology, and therefore its potential to reduce the use of mouse xenograft models.
The study was carried out in collaboration with AstraZeneca, who provided access to five of their novel ADC technologies for these experiments.
Tumour models were developed by first optimising and characterising the conditions necessary for cell engraftment on the chorïoallantoic membrane (CAM) of the eggs using a number of HER2-positive and -negative cell lines. This is important to obtain robust tumour development without deleterious effects on the chicken embryo. On the basis of these studies and to enable comparison with previous studies carried out in mouse models by the collaborator, the HER2-positive cell line NCI-N87 was selected to explore efficacy and toxicity of the ADCs.
Toxicity studies:
The toxicity of cytotoxic pyrrolobenzodiazepine (PBD) warheads were tested alone and bound to a non-HER2 targeting antibody (Neg-ADC) or a HER2-targeting antibody (ADC-01) to determine their effect on chicken embryo development. The maximum tolerated dose (MTD) was found to be 0.25 mg/kg of Neg-ADC, 2.5 mg/kg of ADC-01 and 0.001 mg/kg of PBD warhead alone.
Efficacy studies:
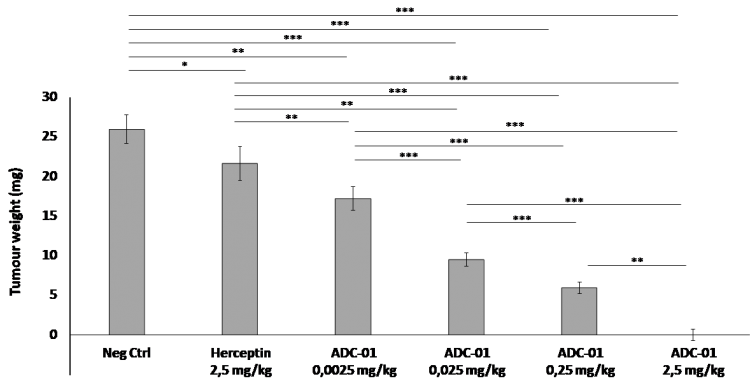
The efficacy of ADC-01 on tumour development was assessed using a range of concentrations from 0.0025 to 2.5 mg/kg (Figure 1). The minimal efficacy dose (concentration for which a significant reduction in tumour weight was observed in comparison to the negative control group that is treated with PBS) was found to be 0.0025 mg/kg for ADC-01, with a 17% regression in tumour size. Total regression was observed at 2.5 mg/kg without toxic effect and was confirmed by histopathological analysis of CAM tissues. For comparison, tumour response in the CAM assay to a clinically relevant dose of Herceptin (the current gold standard treatment) is also shown.
Figure 1. Effect of treatment with different doses of ADC-01 on tumour development.
Comparative efficacy of five ADCs:
The efficacy of four other ADCs using the same antibody, but with different cytotoxic PBDs, was tested at concentrations of 0.25 and 1.25 mg/kg and compared to ADC-01. The efficacy of the ADCs was ranked by comparing tumour weights and metastatic invasion for each group. The ranking from the most to the least efficacious was: ADC-02, ADC-01, ADC-03, ADC-04 and ADC-05. This ranking using the chicken egg model is the same as that observed in the mouse model (in previous studies carried out by Inovotion’s collaborator), demonstrating good correlation. 2.5 mg/kg of ADC-01 and ADC-02 were required for total regression in the chicken egg model (observed 8 days after one treatment), whereas 1 mg/kg was sufficient in the mouse model (observed 21 days after one treatment). This dose difference is likely to be due to the difference of administration (intravenous in mice and peritumoral in ovo) and the limited timeframe for studies carried out in ovo, which require a higher dose to obtain total regression in the time available. The dose of Herceptin used in this in ovo model is clinically relevant and comparable to that found in literature in mouse models (Ko et al, 2015) and human studies (Cameron et al., 2017).
Inovotion has developed a technology that provides fast, sensitive, reliable and affordable assays for a large panel of molecule types. This model could replace mouse studies for the pre-screening of ADCs, in terms of in vivo efficacy and toxicity. This would dramatically reduce the use of rodents, and costs of development, because most candidate molecules will be filtered out prior to other in vivo models. This in ovo test could become a new logical step for drug development, between in vitro and classical in vivo tests. Inovotion is continuing to expand and develop the technology by validating other ADCs and therapeutic antibodies within the model.
References
Ko B-K, Lee S-Y, Lee Y-H, et al. (2015). Combination of novel HER2‐targeting antibody 1E11 with trastuzumab shows synergistic antitumor activity in HER2‐positive gastric cancer. Molecular Oncology.9(2):398-408. doi:10.1016/j.molonc.2014.09.007
Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. (2017).11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet (London, England). 389(10075):1195-1205. doi:10.1016/S0140-6736(16)32616-2