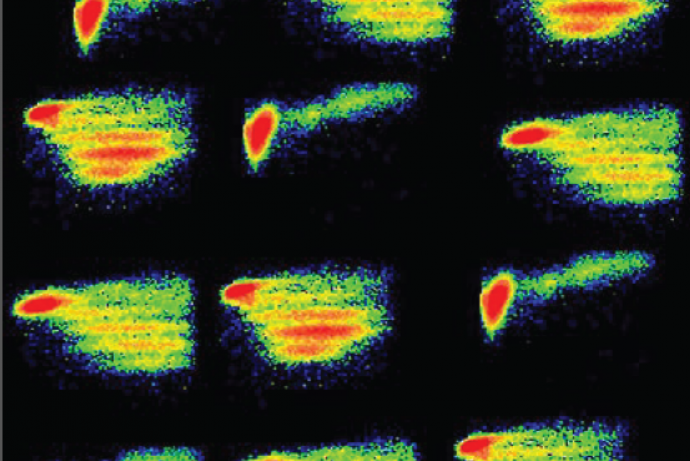
COMPIT is an in vitro platform that enables the comprehensive analysis and prediction of the human immune response to drug molecules. Uniquely, the platform can be used to assess the immune response evoked by cells that have been damaged by drugs. This is neglected in current models for assessing and analysing human immune response. Using human cells, COMPIT has the potential to deliver better translation from preclinical studies to humans and provides users with currently unattainable information on the safety of drugs. Immundnz Ltd are seeking partnerships with companies to help validate the platform in an industrial setting.
Current in vitro approaches for assessing immunogenicity do not compare to the complexity offered by COMPIT. The closest competition provides in vitro cell systems that employ 2D monolayers of primary human lymphocytes, but which are not capable of assessing the immunogenic response induced by drug-damaged tissue (Corsini E et al., 2009). Current immunogenicity testing of drugs considers only the ‘antigenicity’ of the drug molecule, i.e. the ability of a compound to stimulate antibody production, and not the potential impact of tissue damage on the immune response. However, true immunogenicity must also take into account anything that can cause an immune response or generate the conditions to initiate an immune response (e.g. tissue damage). Any drug has the potential to cause unwanted tissue damage which in turn increases the probability of immune-mediated (secondary) pathology (Matzinger, 2002). For example, about 10% of all lupus cases are caused by the unexpected effects of drugs on non-target tissues, including established therapeutics such as streptomycin, adalimumab and etanercept (Hess,1988; Swale et al., 2003).
References
- Corsini E, Roggen EL (2009). Immunotoxicology: opportunities for non-animal test development. ATLA 37(4): 387-397.
- Hess E. (1988). Drug-related lupus. N Eng J Med 318: 1460-1462. doi:10.1056/NEJM198806023182209.
- Matzinger P. (2002). The danger model: a renewed sense of self. Science 12(5566): 301-305. doi:10.1126/science.1071059.
- Swale VJ, Perrett CM, Denton CP et al. (2003). Etanercept-induced systemic lupus erythematosus. Clin Exp Dermatol 28(6): 604-607. doi:10.1046/j.1365-2230.2003.01411.x.
COMPIT is a system of biological and analytical assays that combines novel cell lines, 3D scaffolds and primary cells to deliver an in vitro human cell-based immune system that recapitulates the in vivo human microenvironment. This offers researchers an advanced tool for analysing and predicting the human immune response that goes beyond the capability of currently available approaches.
Immundnz have demonstrated that 3D tissue models, which are more physiologically relevant in vivo cell structures, facilitate drug absorption and lymphocyte infiltration to the site better than flat 2D cultures. An additional advantage of using 3D models is that they are more amenable to histological study, providing additional data to support decision making (Ranga et al., 2014).
It is the immunogenicity of a drug that COMPIT has been specifically designed to assess by dissecting:
- How the biomolecule is processed by the immune cells and the subsequent biological events that follow.
- If the biomolecule causes tissue injury.
- If such events have an impact on the microenvironment to the extent that it might overrun normal adaptive processes and induce an immunogenic response (i.e. change from ‘tolerance’ to immunity).
Using a collection of endpoints, COMPIT provides a novel, ultrasensitive analytical approach to measure the low levels of immune response biomarkers that are generated in these instances, to determine an immune phenotype. Currently available assays are not sensitive enough to measure these biomarkers and so they remain undetectable.
COMPIT is applicable to small and large molecule drugs. With the growing interest in large molecule drugs, there is an increasing need for better understanding the human immune response to these therapeutics. COMPIT will meet this demand from the (bio)pharmaceutical industry in the following ways:
- Drug discovery groups: to assess drug-induced tissue damage and immunogenicity and to predict drug antigenicity during preclinical development.
- CROs: to predict drug antigenicity (anti-drug antibodies) and in toxicological analysis.
- Biosimilars groups: to assess immunologic comparability of biosimilars with reference products, and in the assessment of which drugs pose an immunologic risk to patients/individuals that belong to certain high-risk immune phenotypes.
Reference
- Ranga A, Gjorevski N, Lutolf MP, et al. (2014). Drug discovery through stem cell-based organoid models. Adv Drug Del Rev 69: 19-28. doi:10.1016/j.addr.2014.02.006.
COMPIT is a highly interesting platform for pharma and biotech companies and biosimilar developers as it addresses the demand for an in vitro model to predict human immune response to drugs (Whitebread et al., 2005; Challenge 4: Cytokines, 2011). Crucial to adoption will be to show proof of principle with relevant drug molecules, and so Immundnz are seeking (bio)pharmaceutical partners able to provide access to a range of compounds to validate this novel model. Ideally this would include marketed compounds and molecules that failed due to immunogenicity (with associated data) according to current methods to show the clear and distinct benefits of the COMPIT platform.
Immundnz are also seeking advice and guidance on the development and application of COMPIT in an industrial setting.
Information about IP
COMPIT is in the process of being patented
References
- Whitebread S, hamon J, Bojanic D, et al. (2005). In vitro safety pharmacology profiling: an essential tool for successful drug development. Drug Discov Today 10(21): 1421-1433. doi:10.1016/S1359-6446(05)03632-9.
- Challenge 4: Cytokines. CRACK IT (2011). Available from: https://www.crackit.org.uk/challenge-4-cytokines.
Preclinical studies are performed in animals. Classically, non-transgenic mice, rats and non-human primates are used for immunogenicity studies. A typical study consists of 60 experimental animals per compound to be studied, including tests such as lymph node assays, histopathology, and determination of antigen-specific and auto- antibodies. Such animal models are in many cases not relevant for translation to humans, especially in the case of studying immune response. There is a pressing market and regulatory need for more physiologically relevant in vitro approaches to reduce reliance on these in vivo models (FDA, 2002; FDA, 2014). COMPIT can robustly predict the human immune response, offering a viable alternative approach to replace some animal studies in immunogenicity testing. Additionally, by enabling the comprehensive analysis and prediction of the human immune response to drug molecules in vitro, COMPIT will reduce the number of compounds entering regulatory animal studies and therefore the number of animals used overall.
References
- US Department of Health and Human Services, Food and Drug Administration (FDA). Guidance for Industry: Immunotoxicology evaluation of investigational new drugs. 2002.
- US Department of Health and Human Services, Food and Drug Administration (FDA). Guidance for Industry: Immunogenicity assessment for therapeutic protein products. 2014.