EASE
The aim of this Challenge was to generate an approach that improves the implantation rates of early stage embryos when combined with extended in vitro culture and non-surgical embryo transfer techniques. Using surgical procedures, implantation rates for unmanipulated two-cell embryos are currently ~40%. However, micromanipulated embryos are less robust with ~25% currently giving rise to live offspring.
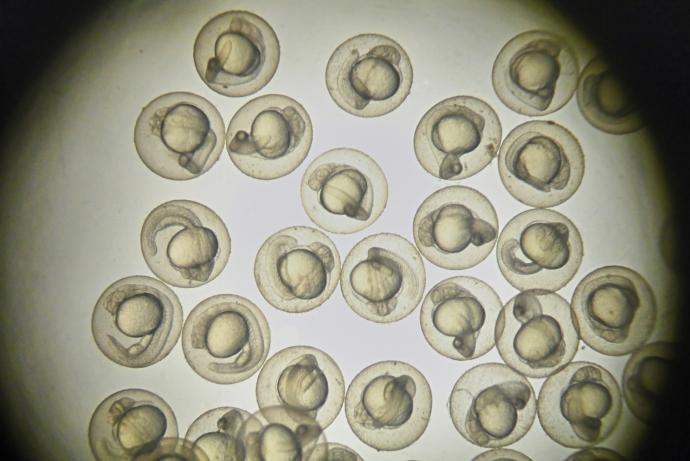
The team at University of Leeds led by Dr Virginia Pensabene has developed a novel and reliable microfluidic device that improves the developmental competence of in vitro-derived mouse embryos to allow the use of non-surgical embryo transfer (NSET) in the generation of transgenic mice.
This project and its impacts are featured as a case study in the 2019 CRACK IT Review.
Investment secured
IVFmicro has received £3.5M for its next verification and validation phase, enabling further trials for its potential application in fertility clinics.
The pre-seed funding is led by Northern Gritstone with support from the Innovate UK Investor Partnerships Programme.
10 years of CRACK IT webinar: Using microfluidics to eliminate surgical embryo transfer in mice

In October 2021, we held a webinar highlighting the IVF micro microfluidic device, developed to address the EASE Challenge, which enables non-surgical embryo transfer for generating transgenic mice. Speakers included Dr Virginia Pensabene (University of Leeds), who led the team that delivered the Challenge, and Sarah Hart-Johnson (The Francis Crick Institute), who is working with the team to further validate and deploy the IVF Micro device. The recording of the webinar is now available online, as part of our 10 years of CRACK IT celebrations.
Publication
Mancini V et al. (2021). Metabolomic Analysis Evidences That Uterine Epithelial Cells Enhance Blastocyst Development in a Microfluidic Device. Cells 10(5): 1194. doi:10.3390/cells10051194
Challenge completed
The team at University of Leeds led by Dr Virginia Pensabene has developed a novel and reliable microfluidic device that improves the developmental competence of in vitro-derived mouse embryos and their implantation potential, enabling the use of non-surgical embryo transfer (NSET) in the generation of transgenic mice.
Poster Award
Society for Reproductive Investigation In Training Investigator Poster Award, Paris 2019
Effects of Uterine Cells-Conditioned Media on Expression of DNMT3B and DNMT3C in Mouse Embryos Cultured in a Microfluidic Device.
Postdoctoral Scientist Prize
Fertility 2019 (Birmingham, UK)
The metabolic and developmental impact of murine embryo culture in a novel microfluidic device.
Conference presentation
Fertility 2019 (Birmingham, UK)
On-chip mouse embryo culture: evaluation of effects of uterine cells-conditioned media on embryo development and gene expression.
International Association of Advanced Materials (IAAM) Scientist Medal
European Advanced Materials Congress (Stockholm, Sweden)
Innovative microfluidic approaches in reproductive biology.
Conference presentation
European Society of Human Reproduction and Embryology Campus symposium (Edinburgh, UK)
Microfluidic Model of the Human Endometrium.
Conference presentation
40th International Engineering in Medicine and Biology Conference (Honolulu, Hawaii)
Mouse embryo assay to evaluate polydimethylsiloxane embryo-toxicity.
Conference presentation
34th Annual Meeting of the European Embryo Transfer Association (Nantes, France)
The use of a novel microfluidic culture device and predictive metabolic profiling as a means to improve murine embryo developmental competence in vitro.
Conference presentation
Organ-on-a-chip TTL launch meeting (London, UK)
Investigating the correlation between infection and preterm birth in a human endometrium-on-a-chip.
Challenge awarded
A team led by Dr Virginia Pensabene from the University of Leeds has been awarded £95,883 to deliver the project: Design, Fabrication and Testing of a Mouse Embryo Culture Chip.
Challenge launched
Sponsored by MRC Harwell, the EASE Challenge aims to generate an approach that improves the implantation rates of early stage embryos when combined with extended in vitro culture and non-surgical embryo transfer techniques. Using surgical procedures, implantation rates for unmanipulated two-cell embryos are currently ~40%. However, micromanipulated embryos are less robust with ~25% currently giving rise to live offspring.
Background
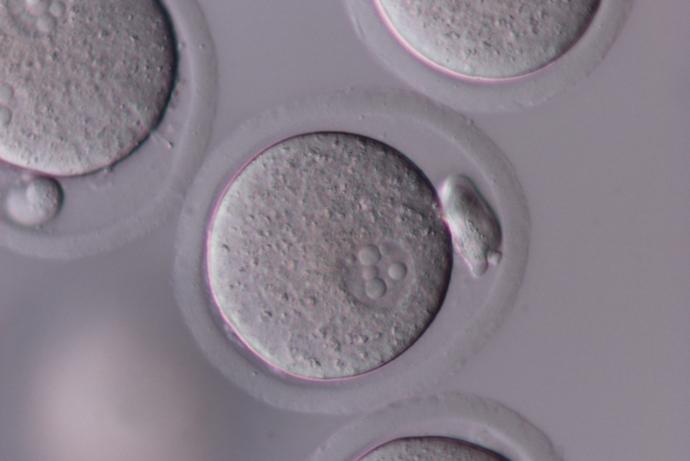
Genetically altered (GA) mice are used extensively to study the function and regulation of genes and their role in human development and disease. In 2015, approximately 50% of the animals used for scientific procedures in the UK were for the creation and breeding of genetically modified animals, the majority of which are mice (Home Office, 2015).
The generation of GA mouse models involves one of three techniques that use embryos at different stages of development:
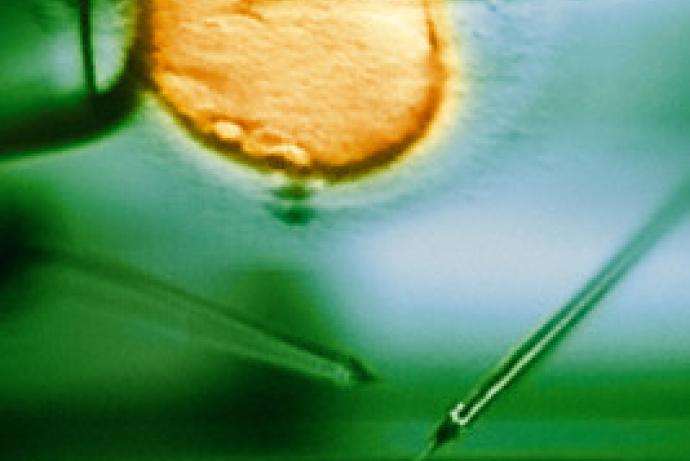
- Pronuclear injections take advantage of one-cell embryos, where DNA or RNA is injected directly into the pronucleus of a fertilised egg. This technique allows scientists to use gene editing techniques like CRISPR/Cas9.
- In vitro fertilisation (IVF) is used to produce two cell embryos. This is a particularly useful technique because it allows scientists to archive or exchange mouse strains as sperm when it is appropriate to do so.
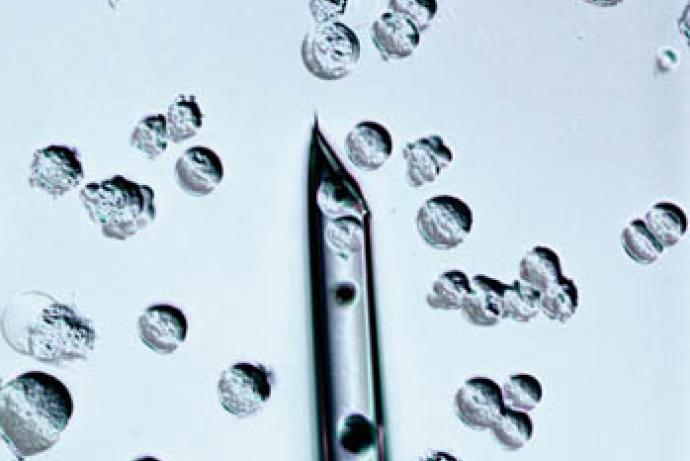
- Embryonic stem (ES) cell injection takes advantage of ES cell culture techniques that allow complex gene targeting events to be performed in vitro. Quality Control (QC) verified ES cells are injected into blastocysts (3.5 day old embryos).
Embryos from all three approaches are transferred into pseudopregnant recipients. The majority of embryo transfer procedures performed in mice use surgical techniques, with implantation into the oviduct or uterus via a laparotomy performed under general anaesthesia (Nagy et al., 2014). Although this technique is well understood and relatively high implantation rates can be achieved, it is an invasive procedure and can lead to discomfort and surgical complications. In recent years, efficient Non-Surgical Embryo Transfer (NSET) techniques have been developed (Steele et al., 2013; Cui et al., 2014) and specific transfer devices are now commercially available.
The NSET approaches pass a catheter through the cervix, allowing embryos to be deposited directly in the uterine cavity. No surgery is involved, offering significant welfare gains. In competent hands these devices work as well as surgical embryo transfer techniques. However, the NSET techniques can only be used to transfer late stage pre-implantation embryos (i.e. the blastocyst stage (E3.5)). This makes the technique suitable for transferring embryos after ES cell injection but it is not suitable for earlier embryonic stages including one and two-cell embryos generated from IVF and pronuclear injection programmes which still need to be transferred into the oviduct using surgical techniques.
Past experience has shown that extended culture of one and two-cell embryos generated from IVF programmes or following pronuclear injection severely compromises their implantation success. This means that these common techniques do not get used in conjunction with the current NSET systems. However, optimising embryo culture conditions is an area of intense research in both the mouse and clinical field. For example, a recent publication by Truong et al., has reported that adding antioxidants (e.g. acetyl-L-carnitine, N-acetyl-Lcysteine and α-lipoic acid) increases blastomere numbers in in vitro cultured embryos, as well as improving fetal development with increased crown-rump lengths and fetal weights. These data indicate that altering the redox potential of embryo culture systems may be advantageous when culturing embryos, prior to NSET. Other work has also shown that the addition of myo-inositol, a precursor of phosphoinositides, also significantly increases the number of blastomeres in embryos that had been produced by a microinjection technique called intracytoplasmic sperm injection (Colazingari et al., 2014).
The aim of this CRACK IT Challenge is to maximise the use of the NSET technique either by the development of a reliable system for culturing in vitro manipulated embryos through to the blastocyst stage or the development of an approach to non-invasively manipulate the uterine environment.
3Rs benefits
Developing a robust system that combines an improvement in in vitro embryo culture conditions with the use of contemporary NSET techniques would eliminate the need to use surgical embryo transfer when implanting one and two-cell embryos. This would have significant benefits to all of those involved in the development of GA mouse models in terms of improved animal welfare and reduced costs.
Removing the need for surgical embryo transfer would eliminate a significant number of invasive procedures. Based on a survey completed by the International Society for Transgenic Technologies between September 2008 and September 2009 (Fielder et al., 2010), it is estimated that in excess of 250,000 surgical embryos transfers are currently performed globally each year. The MRC’s Mary Lyon Centre performs in excess of 2,500 surgical embryo transfer procedures per year. With a viable alternative to surgical transfer of one and two-cell embryos, up to 90% of all invasive embryo transfer procedures could be substituted with a more refined technique.
The availability of techniques developed through this Challenge would also benefit other ongoing research efforts. For example, in recent years the GA mouse breeding community has made huge improvements in the way it exchanges (Kenyon et al., 2014) and stores mouse strains in repositories such as the European Mouse Mutant Archive (Guan et al., 2014). In both cases the preferred option is to use frozen sperm which needs to be recovered using IVF techniques to produce two-cell embryos.
The advent of new gene editing techniques like CRISPR/Cas9 also means that many transgenic laboratories have switched their activities away from ES cell/blastocyst injection and are now concentrating on one-cell injection techniques. This has the potential to increase the number of surgical embryo transfers performed unless NSET techniques can be optimised.
Challenge winner
Project team led by:
Full Challenge information
The team at University of Leeds led by Dr Virginia Pensabene developed a novel and reliable microfluidic device that significantly improves the developmental competence of in vitro-derived mouse embryos and their implantation potential, enabling the adoption and the efficiency of non-surgical implantation (NSET). Using microfluidics allowed Dr Pensabene to control specific features of cell culture (cell position, flow, mechanical cues), and provide unprecedented flexibility in dissecting the cellular, molecular, chemical and physical factors which contribute to the development and function of embryos.
The project coupled expertise in microfluidic, microfabrication technology and reproductive biology (Dr Pensabene) with the extensive experience of Professor Helen Picton in IVF and embryology to create a fully functional microfluidic system (Video 1). The microfluidic device reduces embryo exposure to in vitro stressors and improves the health and competence of manipulated embryos through to the blastocyst stage thanks to a more precise control of media volume, pH and oxygen tension.
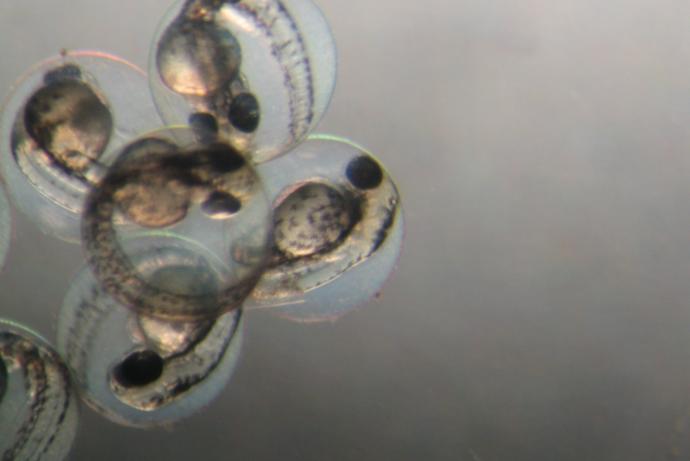
Video 1. One or two cell stage embryos are loaded in the microfluidic chamber by injecting them through the access port.
In house validation of the device and approaches by MRC Harwell has shown maximisation of the blastocyst rate (100%), improved birth rates (53%) when transferring embryos cultured for 48hrs in the devices with surgical procedures. When combined with NSET, the microfluidic culture ensured successful implantation of in vitro matured blastocysts (success rate >25%). This technology eliminates the current standard use of potentially toxic mineral oil for embryo culture (thus reducing toxicity, failure, £0.32 cost for each culture dish) and favours the adoption of NSET. Using the microfluidic dish in combination with NSET, animal breeding labs will eliminate the costs related to bench and animal preparation for surgery (setting up, packaging, sterilisation and cleaning of surgical tools, health monitoring, anaesthetics, analgesics, etc.), reduce the times required for embryo transfer (from one hour to 15 min) and for training lab technician (from four months to one month).
For further information please contact Dr Virginia Pensabene
IVF Micro – in vitro microfluidic embryo culture
A team led by Virginia Pensabene at the University of Leeds have developed the IVF Micro system that supports the culture of mouse embryos, from single cell through to blastocysts. The unique approach aims to improve the implantation rates of early stage embryos, combining long-term in vitro culture and non-surgical embryo transfer techniques (NSET) in the generation of transgenic mice.
The IVF Micro microfluidic system has advantages compared to standard dish culture:
- Mimics the embryo environment in vivo;
- Eliminates the use of oil, reduces nutrient stress and better supports the embryo development;
- Reduces handling and exposure to stressors (i.e. shear stress and pH changes).
The solutions developed will help improve the success of Assisted Reproduction Technologies (ART) in animals and humans by enabling safer, simpler, and more reliable in vitro culture for high quality embryos.
The original project and its impacts are featured as a case study in the 2019 CRACK IT Review.
Features



Access the technology
To find out more about this product and how to acces it, please contact Dr Virginia Pensabene or visit the IVF Micro website.
References
- Mancini V, McKeegan PJ, Schrimpe‐Rutledge AC, Codreanu SG, Sherrod SD, McLean JA, Picton HM, Pensabene V. (2021). Probing morphological, genetic and metabolomic changes of in vitro embryo development in a microfluidic device. Biotechnology Progress. https://doi.org/10.1002/btpr.3194
- Mancini V, Schrimpe-Rutledge AC, Condreanu SG, Picton H, Sherrod SD, McLean JA, Pensabene V. (2021). Metabolomic Analysis Evidences That Uterine Epithelial Cells Enhance Blastocyst Development in a Microfluidic Device. Cells. 10(5), 1194; https://doi.org/10.3390/cells10051194