Researchers at the University of Naples have developed a liver-on-a-chip platform composed of 3D human hepatocyte-like engineered microtissues dynamically cultured in a microfluidic device, to more closely mimic the human in vivo environment. The system could be used to investigate the safety (hepatoxicity) and efficacy of chemicals and drugs, as well as the potential protective properties of nutraceuticals. Industrial partners involved in drug development and screening able to provide compounds and data are sought to help develop and validate the model as an effective tool for drug development. Partnerships are also sought with the food industry to test nutraceuticals.
During drug development the candidate molecule is fully characterised for its absorption, distribution, metabolism, excretion (ADME) and toxicity properties. The liver plays an important role in drug metabolism and is susceptible to injury, making drug-induced hepatoxicity a leading cause of attrition during preclinical and clinical drug development. Animal models do not always accurately predict effects in humans due to species-specific differences in drug metabolism pathways. In vitro human liver models are used as an early screen to predict drug-induced hepatoxicity and to understand drug-metabolism in humans. Conventional in vitro models of the liver are based on static 2D human hepatocyte cultures (e.g. primary human hepatocytes or immortalised cell lines), however, these models have several limitations, for example, cells fail to maintain differentiation and expression of tissue-specific functions, express low levels of drug metabolism enzymes and do not recapitulate the complex 3D tissue architecture (Ramaiaghari et al., 2014).
Human liver-on-chip platforms (defined as 2D or 3D hepatocyte or co-culture models cultured within a device exposed to dynamic flow) have been developed to more closely mimic the in vivo environment. The liver-on-chip platforms that use 2D hepatocytes or co-cultures fail to recapitulate the 3D physiological environment with its dynamic complexity (Lee et al., 2007; Ho et al., 2013). Others have cultured cells within a 3D scaffold, however, the cells grow as a layer on top of the scaffold, losing the advantage of the 3D culture setting (Esch et al., 2015). Other groups have created 3D human hepatocyte spheroids using extracellular matrices such as Matrigel and have shown enhanced biological properties over 2D static cultures (Ramaiaghari et al., 2014). However, spheroid models have limitations including difficulty in controlling aggregate size, and spheroids exceeding 200 microns in size suffer oxygen limitation and necrosis at the core. In addition, while spheroid configuration guarantees cell-cell interaction, it does not preserve the cell-extracellular matrix (ECM) interplay resulting in a 3D tissue construct in which metabolic activity, mechanical properties and ECM composition do not resemble the in vivo setting. Therefore, more physiologically relevant and reliable human in vitro models to predict hepatotoxicity are required.
References:
- Esch MB, Prot JM, Wang YI, et al. (2015). Multi-Cellular 3D Human Primary Liver Cell Cultures Elevate Metabolic Activity Under Fluidic Flow. Lab on a Chip 15(10): 2269–2277. doi: 10.1039/C5LC00237K.
- Ho CT, Lin RZ, Chen RJ, et al. (2013). Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab on chip 13(18): 3578–3587. doi: 10.1039/c3lc50402f.
- Lee PJ, Hung PJ, Lee LP (2007). An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnology and Bioengineering 97(5): 1340–1346. doi: 10.1002/bit.21360.
- Ramaiahgari SC, den Braver MW, Herpers B, et al. (2014). A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Archives of Toxicology 88(5): 1083–1095. doi: 10.1007/s00204-014-1215-9.
To overcome the limitations of static 2D human hepatocyte cultures, a liver-on-a-chip platform has been developed, composed of 3D human hepatocyte-like engineered microtissues dynamically cultured in an optically accessible lobule-like microfluidic device, providing a more physiologically relevant environment (Figure 1 and 2).
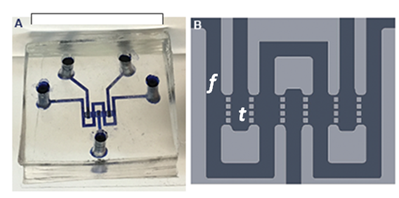
Figure 1. Liver biochip design. The biochip is designed to mimic the hepatic sinusoids. The circuit consists of three parallel tissue chambers (t) separated from a middle channel (f) by small pillars. The small horizontal channels between the pillars mimic the sinusoids.
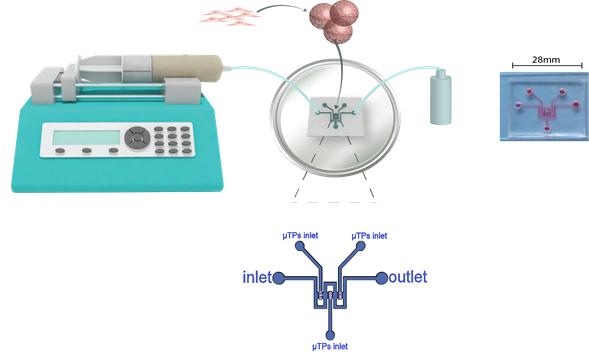
Human liver-like 3D microtissues (HepG2-μTPs 300-400 μm in size) are produced by seeding HepG2 cells onto gelatin porous microcarriers and cultured in spinner flask bioreactors. HepG2-µTPs show stable viability, morphology (cuboidal shape and tight junctions), function (albumin and urea synthesis), metabolic activity and Pgp transport protein expression measured over 14 days of culture in spinner flask bioreactors. After 5-7 days, HepG2-µTPs are collected from the bioreactors and cultured in a microfluidic device designed to mimic the hepatic sinusoids under continuous fluid flow (Figure 1 and 2). Microtissues maintain stable viability and function over 4 days of culture within the device. Culturing the microtissues in the device does not affect their morphology, function and metabolic activity allowing the full potential of the system to be exploited, i.e. the suitability to be used for high throughput toxicity studies. This initial characterisation provides evidence that the system offers a physiologically relevant platform to test the safety (drug-induced hepatotoxicity) and efficacy of new pharmaceutical compounds.
Figure 2. Schematic representation of the microfluidic biochip set-up. 3D liver microtissues after ~ 6 days of culture in a spinner flask bioreactor are loaded into the microfluidic chip. Culture medium is then infused through the middle channel using a syringe pump and supernatant is collected at the outlet. Fluid velocity is set on the syringe pump controller at a flow rate of 5 μl min-1.
The main features and advantages of these 3D human hepatocyte-like micro-tissues that more closely mimic the in vivo environment are:
- Compounds can be tested over several days.
- Potential to co-culture microtissues with non-parenchymal liver cells (e.g. Kupffer cells, stellate cells or sinusoidal endothelial cells) due to the device geometry.
- Easy access for non-invasive morphological and functional analyses due to the optical accessibility of the chip over the entire culture time.
- Easy to set up and handle for routine drug screening decreasing the risk of contamination during both test molecule administration and sample withdrawal thanks to the chip structure.
- Cost-effective.
This Solution could also be applied to other industry sectors, such as the chemicals sector where large numbers of animals are used for toxicology screening under the REACH (Registration, Evaluation, Authorisation and restriction of Chemicals) regulations, and to the food industry to test nutraceuticals. The application of the platform could also be expanded into other research areas, for example a combined liver and gut on-a-chip platform to study first-pass metabolism.
An industrial partnership is sought to further develop/characterise and validate the device to expand its use beyond scientific purposes into application to test new molecules, active principles or drugs. Therefore, partners from the biotechnology and pharmaceutical industry able to provide advice, compounds, and preclinical (e.g. in vitro data from 2D hepatocyte cultures) and clinical datasets for comparison, are sought to validate the model for drug safety (hepatotoxicity) and efficacy screening. Partnerships are also sought with food companies to provide nutraceuticals to test in the system.
In addition, partnerships are sought to help further develop and validate the device with microtissues composed of hiPSC-derived liver cells (and/or primary human hepatocytes), and/or to co-culture human hepatocyte-like microtissues with non-parenchymal cells (e.g. Kupffer cells, stellate cells, sinusoidal endothelial cells).
In vivo assessment of drug-induced hepatoxicity in rodent and non-rodent toxicology studies typically involves evaluating liver histopathology along with measuring clinical chemistry markers such as alanine aminotransferase and aspartate aminotransferase. These studies use large numbers of animals, are low throughput, costly, time consuming, invasive and are not always predictive of effects in humans due to species-specific difference in drug metabolism pathways. A rodent (rat) repeat-dose GLP toxicology study uses up to 200 animals per study (e.g. three dose groups and a control group (up to 15 animals per sex per dose group), plus recovery animals (five animals per sex for control and high dose group) and toxicokinetic satellite animals) (Sparrow et al., 2011). If hepatoxic signals are identified in these studies then this will trigger follow-up studies to characterise the hepatoxic effects and identify possible mechanisms, further increasing the number of animals used.
This Solution has the potential to reduce animal use by providing a screen to improve the early identification of drugs with undesirable properties, preventing these drugs from progressing into animal studies. In the longer term, as more data are generated and the system is developed and validated further and shown to be predictive of effects in humans, the Solution may provide an absolute replacement of animal models.
In addition, application to other industrial sectors (e.g. chemical and food industry) and future development of the system into other research areas will further increase the potential 3Rs impact.
References:
- Sparrow S, Robinson S, Bolam S, et al. (2011). Opportunities to minimise animal use in pharmaceutical regulatory general toxicology: A cross-company review. Regulatory Toxicology and Pharmacology 61(2): 222-229. doi: 10.1016/j.yrtph.2011.08.001.