ImmuLiver
The aim of this Challenge was to develop an in vitro model of the human liver that is metabolically and immunologically-competent for the routine assessment of Yellow Fever (YF) vaccine attenuation.
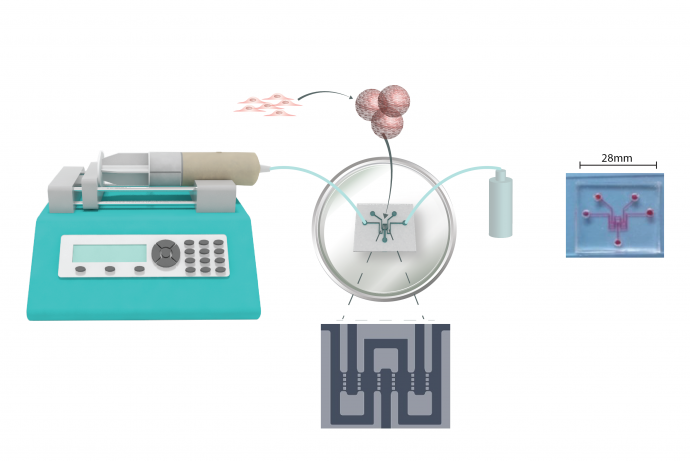
To address the ImmuLiver Challenge, a team led by Professor Lyle Armstrong, Newcells Biotech Ltd have developed a human induced pluripotent stem (hiPS) cell-derived 3D liver sinusoid model to recapitulate the structure of the human liver.
Challenge Completed
Through the ImmuLiver Challenge the team led by Professor Lyle Armstrong, Newcells Biotech Ltd have developed a human induced pluripotent stem (hiPS) cell-derived 3D liver sinusoid model to recapitulate the structure of the human liver.
Phase 2 awarded
A team led by Professor Lyle Armstrong from Newcells Biotech Ltd has been awarded £957,793 to deliver Phase 2.
Phase 1 awarded
Three Phase 1 Awards were made to project teams led by:
- Dr Marcus Dorner, Imperial College London, £100,000.
- Dr Ruchi Sharma, Stemnovate Ltd, £99,960.
- Professor Lyle Armstrong, Newcells Biotech Ltd, £99,711.
Challenge launched
Sponsored by Sanofi Pasteur EU, the ImmuLiver Challenge aims to develop an in vitro model of the human liver that is metabolically and immunologically-competent for the routine assessment of Yellow Fever (YF) vaccine attenuation. The vaccine is based on a live-attenuated virus derived from a pathogenic strain of YF virus and is currently assessed in non-human primates (NHPs), specifically cynomolgus macaques. Existing in vitro liver models are not fit for viral testing in a pharmaceutical context due to a lack of immune competency. A non-exhaustible or easily renewable source of cells, with a characterised origin is required. The model must be compatible with automated distribution and multiple sample analysis, as well as the constraints linked to manipulations of pathogenic agents (Biosafety Levels 2 and 3 (BSL2, BSL3))
Background
Yellow fever is a mosquito-borne flavivirus disease affecting human populations in tropical areas of South America and Africa. The virus maintains an enzootic cycle in NHPs and is periodically re-introduced to humans through infected NHPs living nearby (Fernandes et al., 2017; Moreno et al., 2013). This means the global eradication of Yellow Fever virus (YFV) infection is not possible and it remains a major public health concern. YFV infection can cause severe illness characterised by the impairment of liver function, leading to acute hepatitis, severe hemorrhage and if it proceeds to infect the central nervous system, death (Monath, 2001; Monath et al., 2015). YFV-infected cynomolgus macaques fully recapitulate the pathophysiology of human YF disease, and were central in the development of the first YFV vaccine strain, YF17D (Frierson JG, 2010), obtained by the prolonged cultivation of the wild type YFV-Asibi strain.
For the development and evaluation of vaccines, current regulatory guidelines (WHO Annex 5, 2013) recommend that appropriate safety characterisation studies should be conducted using the YF17D vaccines as a comparator. Due to the lack of suitable small animal models able to mimic YF pathophysiology, much weight is currently placed on cynomolgus macaque neurovirulence studies for vaccine safety evaluation (Levenbook et al.,1987). A typical safety test is described in “Recommendations to assure the quality, safety and efficacy of live attenuated yellow fever vaccines” (WHO Annex 5, 2013).
In this assay, neurovirulence, immunogenicity and viscerotropism are evaluated in the same macaques (n=10) through innoculation with a test dose in the frontal lobe followed by clinical observation, sampling and post-mortem histology. Neurotropism can also be evaluated through peripheral innoculation.
There is strong industry interest in replacing in YFV vaccine attenuation assessment.
- Neurovirulence/ neurotropism: a recent pilot study conducted by Sanofi Pasteur, with wild type and attenuated YFV vaccine has shown that in vitro blood/brain barrier (BBB) mini-brains constitute a promising alternative to macaques for neurovirulence testing with the additional ability to detect neurotropism (da Costa et al., 2018).
- Immunogenicity: studies can be performed in small rodent animal models and when combined with the product of this Challenge and the in vitro BBB assay developed by Sanofi, could lead to the replacement of studies in macaques.
- Viscerotropism: is currently assayed by measuring by blood viraemia in the innoculated macaques. An in vitro model, as outlined in this Challenge, has the potential to replace this assay.
An in vitro model of viscerotropism
The development of an immune competent in vitro liver model for use in the viscerotropic assessment of YF vaccine products has the potential, when combined with the in vitro neurovirulence model, to replace the use of macaques in safety testing of these products. The use of small animal models for assessment of viscerotropism is limited by their poor predictivity. For example, type I Interferon (IFN)-deficient mice succumb to infection with wild type virus and survive infection with the vaccine, but are immuno-compromised, and hamsters can develop viscerotropic symptoms and high levels of virus in the liver, but only when infected with a hamster-adapted virus strain (Julanger, 2016). These have limited predictive value for viscerotropism and a human relevant in vitro model would have significant potential to replace the use of macaques.
Both the 17D vaccine and wild type (Asibi) viruses can productively infect various liver cells in vitro and elicit similar replication levels, making them indistinguishable by replication when used in the same model. However, Sanofi have recently demonstrated that human primary hepatocytes may be a valuable in vitro model to investigate innate and metabolic disorders elicited by YFV infection. The study was conducted in a commercial human liver organotypic model (InSphero™) based on primary hepatocytes. Using a transcriptomics screen, type III IFN was identified as a potential biomarker to differentiate between wild type and vaccine YFV. This observation is consistent with other reports describing the critical role of type III IFN in clearance of hepatic infections, like hepatitis C, in vitro and in vivo. Other potential biomarkers have been identified, suggesting that such an in vitro model could constitute an alternative to the macaques viscerotropic assay to predict YF liver disease (Masse-Deragon et al., 2016, thesis work and manuscript in preparation).
A functional liver model that can recapitulate as much as possible the complexity of the organ, is needed to assess viscerotropism and replace macaques in the testing of new vaccine products. Besides its major role in metabolism, the liver is also a critical component of defence against blood-borne infections (Crispe, 2009). It contains both conventional and unconventional populations of resident antigen-presenting cells (APCs), like Kupffer cells, liver sinusoidal endothelial cells and hepatic stellate cells, which can support T cell activation. The presence of such cells in a model of liver infection is critical. 3D cell culture has been demonstrated to recreate the in vivo microenvironment and physiological relevance of tissues (Mehta et al., 2012). A number of multicellular liver models are commercially available but have not been proven to be suitable for routine assessment of YFV vaccines. The principal limitations are:
- A lack of well-defined models: there is no definition for “normal” functioning for a liver immune competent model, and a lack of biological markers to assess this status.
- A limited supply of primary cells makes them incompatible with routine testing for the lifetime of the vaccine (usually >30 years) and they are usually isolated from donors with a medical history of liver pathology (diabetes, cancer, alcohol abuse). The impact of the donor status may impact the outcome of an infection study.
- Culture medium formulations and extracellular matrix (ECM) components are not optimised for viral infection (e.g. interference with receptor fixation, reduced virus half-time, viral particle dissociation).
- Design of the models does not always permit BSL2 safety containment of the pathogen (protection of the operator and the environment, safe elimination of liquid and solid wastes).
- Insufficient assay throughput and limited number of readouts for assay validation.
3Rs benefits
All currently manufactured YF vaccines are based on two subtypes of the YF17D attenuated strain. WHO current guidelines recommend that attenuation in macaques is assessed for each new manufactured seed lot and for any new YFV attenuated strain (WHO Annex 5, 2013). A typical assay is described in Moulin et al (Moulin et al., 2013). Depending on the manufacturing capacity, this assay is repeated two to four times a year. For each new working seed lot, at least two groups of ten cynomolgus macaques (both sexes), one test group and one reference group, are anesthasised and inoculated with viral suspension intracerebrally into the frontal lobe. Macaques are observed for 30 days and records of clinical observations (ranging from not eating to limb paralysis and death) are obtained according to WHO recommended scores.
With growing evidence that both the neurovirulence assay will be replaced by an in vitro assay in the future (da Costa et al., 2018), there is an urgent need to find a substitute to the macaque viscerotropism test to enable replacement of this invasive and terminal procedure. A robust, biologically-relevant in vitro assay for vaccine attenuation assessment would provide a strong case to convince health authorities to remove the macaque assay from the YF vaccine guidelines. As an example, the US and Canadian Health Authorities (FDA and BGTD, respectively) have recently approved the replacement of the inactivated polivirus (IPV) inactivation test in Primary Monkey Kidney Cells (PMKC), by an in vitro assay in a mouse cell line genetically engineered to express human poliovirus receptor, CD155, based on a robust validated assay (da Costa et al., 2018).
There are many forms of liver infection, classified according to the category of the infecting agent as viral (e.g. cytomegalovirus, HIV and herpes simplex), bacterial (e.g. acute bacterial hepatitis, bacterial liver abscesses and granulomatous liver disease) or parasitic (e.g. plasmodium P.vivax, liver flukes (trematoda) and tapeworms (cestoda)). Implementation in industrial and academic settings, of assays developed through this Challenge could be a viable and improved alternative to the current animal models used for studying these various hepatic pathogenes as well as for the development of vaccines and antivirals.
Phase 1 winners
Project teams led by:
- Dr Marcus Dorner, Imperial College London, £100,000.
- Dr Ruchi Sharma, Stemnovate Ltd, £99,960.
- Professor Lyle Armstrong, Newcells Biotech Ltd, £99,711.
Full Challenge information
Assesment information
A team led by Professor Lyle Armstrong, Newcells Biotech Ltd, in collaboration with Challenge Sponsors Sanofi Pasteur EU, have developed a human induced pluripotent stem (hiPS) cell-derived 3D liver sinusoid model to recapitulate the structure of the human liver.
The project aimed to develop a human-relevant in vitro cell model to assess the safety of attenuated viral vaccines to replace the use of non-human primates in the prediction of the human yellow fever vaccine response. In addition to the routine batch testing, the model may also have scope to test the safety of new vaccine products.
The 3D sinusoid model developed by Newcells Biotech is composed of hiPS-derived hepatocytes, hepatic stellate cells, endothelial cells and immune-competent macrophages that reflect the in vivo complexity of the liver. The team also developed a robust manufacturing process for the sinusoids that facilitated reproducible formation of the 3D model and phenotypic stability of the hiPS cells. Challenge Sponsor Sanofi Pasteur provided in-kind contributions including studies to confirm that the model can recapitulate yellow fever infection, in collaboration with the Liverpool School of Tropical Medicine.
NewCells Biotech Ltd have further developed their liver model, partnering with a consortium led by Gain Therapeutics Inc and with the Institute for Research in Biomedicine (Lausanne) and the University of Helsinki. The consortium received a Eurostars grant of €1.2M to build linked models of the liver and lung as a screening assay system to test the efficacy and safety of novel therapies for AAT deficiency, a genetic disease which causes lung and liver damage.
Further information on the liver models, contact Newcells Biotech Ltd.