Metaboderm
The aim of this Challenge was to establish, both qualitatively (which metabolites are produced) and quantitatively (concentration of the metabolites produced), the extent to which skin metabolism determines xenobiotic availability.
To address this Challenge, a consortium led by Professor Lyle Armstrong, NewCells Biotech Ltd, developed an induced pluripotent stem cell (iPSC) based in vitro skin equivalent and in silico model to investigate the penetration and metabolism of xenobiotic substances on the skin.
Challenge completed

Through the Metaboderm Challenge the team at Newcells Biotech Ltd, led by Professor Lyle Armstrong, developed an induced pluripotent stem cell (iPSC) based in vitro skin equivalent and in silico model to investigate the penetration and metabolism of xenobiotic substances on the skin.
Conference presentation
British Toxicological Society Annual Congress 2018 (Gateshead, UK)
Using statistical models to identify key variables critical for generation of retinal organoids from iPSC for further use in pharmacological and toxicological studies.
Conference presentation
Skin Metabolism meeting 2017 (Sunderland, UK)
Conference presentation
NC3Rs David Sainsbury Seminar Series (Newcastle, UK)
What is the future of 3Rs research?
Phase 2 awarded
A team led by Professor Lyle Armstrong from Newcells Biotech Ltd has been awarded £800k to deliver the project: 3D hiPSC derived skin model and in silico skin modelling of metabolism.
Phase 1 awarded
Three Phase 1 Awards were made to project teams led by:
- Professor Mark Birch-Machin, Newcastle University, £99,999.
- Dr Craig Murdoch, University of Sheffield, £100,000.
- Dr James Sidaway, Phenotox Ltd, £99,730.
Challenge launched
Sponsored by GSK, Dstl, Stiefel and Unilever, the Metaboderm CRACK IT Challenge aims to deliver new tools to understand and interpret human relevant skin metabolism, including rates of metabolism in the skin and metabolite identification.
Background
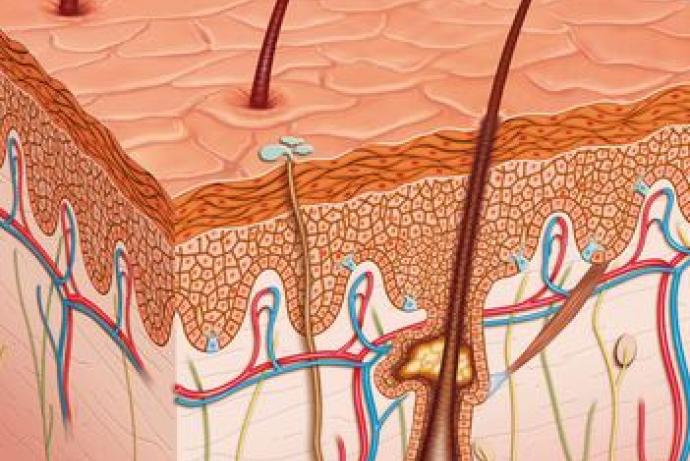
Establishing the absorption, distribution and metabolism properties of xenobiotics in skin is critical in the design and risk assessment of topically applied drugs and excipients, cosmetics for direct application and indirect dermal exposure, and the hazard assessment of chemicals in the environment.
Substantial progress has been made in understanding absorption and distribution of xenobiotics in skin through combined clinical, in vitro and modelling efforts (Mitragoni et al., 2011; Mohammed et al., 2014; OECD, 2004; Roberts 2013). However, the extent to which skin metabolism plays a role in determining local dermal availability of xenobiotics is not well understood (Gibbs et al., 2007). Very little advance has been made clinically and determining metabolic activity in ex vivo human skin has proved problematic due to both lack of sensitivity in measurement techniques and the rapid loss of enzyme activity, even in freshly isolated human skin (Jacques et al., 2014). Consequently, it has been difficult to establish how representative existing results from in vitro 3D skin models might be of metabolism in human skin (Götz et al., 2012a; Götz et al., 2012b). In silico models predicting sites and products of metabolism are predominantly built on liver data and may be of limited relevance for skin. A more useful approach may be to apply the available knowledge on enzymes expressed in skin with docking approaches more specific to the protein-ligand interaction (Kirchmair et al., 2012). Quantitatively, metabolic clearance data is fundamental to understanding xenobiotic availability in major tissues through physiologically based pharmacokinetic (PBPK) modelling, but currently there are no tools available to kinetically model skin metabolism or its impact on xenobiotic availability in skin.
3Rs benefits
For regulatory submissions in the development of drugs for topical administration, a pharmaceutical company will use around 1,000 animals in studies relating to skin toxicity per year- approximately 30% of which involve non-rodent species, in particular, the minipig. While a successful Challenge will not completely eliminate the use of these animals, the novel modelling approaches developed though this Challenge will reduce and replace significant numbers of animals and where animals are still used, minimise the number of required time points/doses. As the market for developing new chemical entities specifically for topical applications is expanding, (Kelly Scientific, 2015), the 3Rs impact of this Challenge will continue to increase.
The development of PBPK models based on dermal exposure is currently an area of active research. Currently, these models may be used in addition to in vivo approaches to predict pharmacokinetic (PK) and toxicokinetic (TK) properties of candidate drugs. A GlaxoSmithKline study reviewing drug attrition between 2007 and 2012 found that around 2% of drugs failed due to skin-related toxicity. This attrition could be prevented by the outcomes of a successful Challenge. Better understanding of skin metabolism will improve dermal PBPK models, enable better selection of chemicals and reduce the use of in vivo toxicokinetic models. A platform which could deliver this would impact animal use across the personal care product, pharmaceutical and agrichemical industries where concerns around skin toxicity exist.
Frequently, in vitro/in silico methods in toxicology aim to reproduce data generated using animal models. The aim of this Challenge is not to predict animal toxicity data but rather focus on safety risk assessment based on data relevant to human use as outlined in Toxicity testing in the 21st century: A vision and a strategy (TT21C) (Krewski et al., 2010). Specifically, the tools developed in this Challenge will allow skin metabolism studies to be conducted without the use of animals and also improve approaches to address the impact of xenobiotic metabolism in skin, informing the understanding of dermal and systemic availability of materials applied to the skin in humans.
Phase 1 winners
- Professor Mark Birch-Machin, Newcastle University, £99,999.
- Dr Craig Murdoch, University of Sheffield, £100,000.
- Dr James Sidaway, Phenotox Ltd, £99,730.
Phase 2 winner
Project team lead by:
Full Challenge information
Assessment information
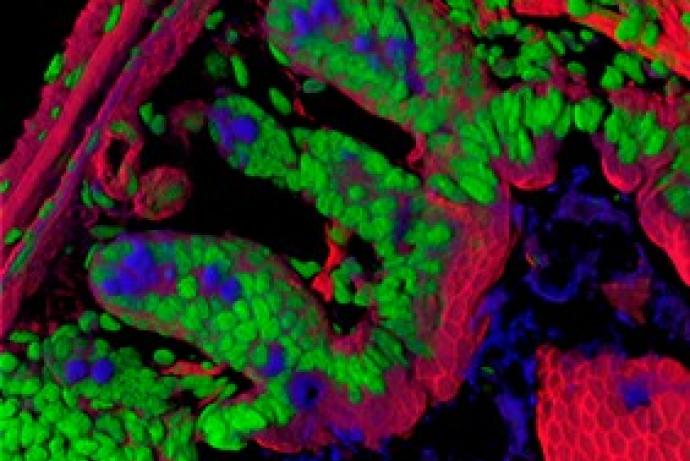
A consortium led by Professor Lyle Armstrong, NewCells Biotech Ltd, with Newcastle University and Sheffield Hallam University has developed an induced pluripotent stem cell (iPSC) based in vitro skin equivalent and in silico model to investigate the penetration and metabolism of xenobiotic substances on the skin.
Using existing analytical and simulation modelling processes, the in silico skin model can accurately predict the metabolism of compounds in skin tissue supported by the iPSC-derived full thickness in vitro skin equivalent. The system could help to determine dosing levels and identify potential toxic responses of cells, tissues and organs to xenobiotic compounds, with applications to the pharmaceutical, cosmetic and consumer goods industries.
The development of these models addresses a critical need for skin metabolism models that can predict the fate of chemicals following topical application. Preclinical regulatory submissions for drugs designed for topical application use both rodent and non-rodent models to assess skin toxicity. Current alternative models are limited, for example the use of ex vivo skin that can be highly variable or in vitro models that do not contain the multiple cell types needed to fully represent skin tissue physiology.
The model systems developed by NewCells Biotech Ltd may reduce the number of animals required in toxicology testing and, where animals are still used, reduce the number of endpoints required in these studies. The implementation of physiologically relevant models may also improve the quality of data from such trials.
The Sponsors, Unilever, GSK and Dstl supported the model development and identified key metabolic pathways of interest. NewCells Biotech Ltd have developed a long-term relationship with the Unilever to explore larger applications of the technology.
For more information about the Metaboderm model, please contact Newcells Biotech Ltd.