Researchers from the Department of Veterinary Sciences, University of Turin have developed acute and organotypically cultured spinal cord slice preparations (SCSPs) to study pain. This ex vivo Solution enables multiple slices to be obtained from each animal, therefore decreasing the number of animals required per study. The researchers are seeking partners to help further develop and validate the innovative SCSPs for studying nociception and neuroinflammation ex vivo, and for preclinical development of novel therapeutics.
With CRACK IT Solutions funding, Professor Merighi is now working with a team led by Professor Eustace Johnson at the University of Chester, UK. Together, they will use the spinal cord slice platforms model to study the effects of transplanting mesenchymal stem/stromal cells into the spinal cord, to better understand their effects on neuroinflammation, central neuropathic pain and neuronal hyperexcitability.
Controlling pain is a major challenge in clinical medicine. Research in the field of pain and neuroinflammation is currently conducted either in vivo or on isolated cells cultured in vitro. Animal models are used to understand the mechanisms of physiological and/or pathological pain and are always associated with severe suffering. These experiments, usually in rodents, not only require inducing pain by different physical or chemical approaches, but also, specifically when neuropathic or chronic pain is studied, induce the generation of nerve injury resulting in long lasting pain hypersensitivity. Although working in vivo offers the obvious advantage of maintaining an intact neuronal circuitry, the difficulties in using animal models to study the circuitry and neurophysiology of nociceptors are widely documented (Mao,2012). These in vivo models are technically very demanding and suffer from a high degree of variability in the nociceptive responses between individuals and sets of experiments. Additionally, drug bioavailability can be difficult to control and certain substances do not pass the blood-brain barrier, thus requiring spinal intrathecal administration, increasing complexity and welfare burden. Many of these shortcomings can be overcome using cultured primary neurons and/or neuronal cell lines, but circuitries are lost by this approach, rendering it unsuitable to the study of cell-to-cell communication and modulation of neural signals.
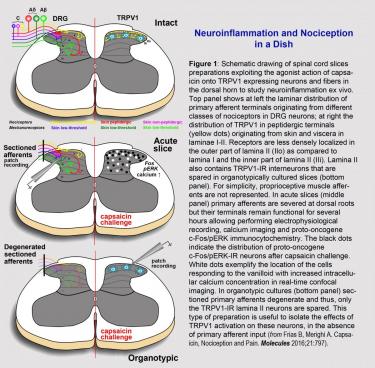
To bypass some of these difficulties, the researchers have developed acute and organotypically cultured spinal cord slices and demonstrated that they can provide unique insight into the central mechanisms of nociception and neuroinflammation. The transient receptor potential cation channel vanilloid subfamily member 1 (TRPV1) is crucial to the onset of inflammation of peripheral tissues. The researchers have characterized the response of spinal cord slices to challenge with capsaicin, a natural agonist of TRPV1 (Ferrini et al., 2014), and demonstrated its usefulness to model acute inflammation in central neurons (Frias and Merighi, 2016).
References
- Ferrini F, Russo A, Salio C (2014). Fos and pERK immunoreactivity in spinal cord slices: Comparative analysis of in vitro models for testing putative antinociceptive molecules. Annals of Anatomy - Anatomischer Anzeiger 196(4): 217-23. doi:10.1016/j.aanat.2013.11.005.
- Frias B, Merighi A (2016). Capsaicin, Nociception and Pain. Molecules 21(6): 797. doi:10.3390/molecules21060797.
- Mao J (2012). Current challenges in translational pain research. Trends Pharmacol Sci 33(11): 568-73. doi:10.1016/j.tips.2012.08.001.
The researchers have developed acute and organotypically cultured postnatal (P7-P10) and young adult (P30) spinal cord slice preparations to study pain. The age of the donor is crucial (Jackson et al., 2016) as the nociceptive system matures around P21 in rodents. The possibility to cultivate both immature and mature tissues offers unique opportunities to study the plasticity of neural circuits and their responses to inflammatory challenges. The principles of the preparation and use of the spinal cord slices as a platform for studying central pain signals are illustrated in Figure 1.
Slices have evolved as the predominant in vitro preparation used by electrophysiologists, and to a lesser extent, histologists, pharmacologists and biochemists, because they retain the cytoarchitecture of the tissue of origin. As slice-based assay systems allow precise control of extracellular environments, they facilitate research aiming to establish clear correlations between structure and function, as well as plasticity of neuronal interactions under different experimental conditions.
The cultures can be subjected to inflammatory challenge and the response of the synapses between first and second order nociceptive neurons monitored by bulk-load calcium imaging of their somas, patch-clamp electrophysiological recordings, or quantification of early gene (fos/erk) activation (Salio et al., 2014). The release of nociceptive mediators can be monitored by immunochemical procedures (e.g. ELISA or immunocytochemistry) (Salio et al., 2014). Notably, the system can be further manipulated by genetic engineering using a physical transfection method (gene-gun) that, at least in part, avoids the need to generate novel transgenic strains, further reducing the use of animals in these studies. The researchers have developed an ex vivo FRET-based procedure that monitors caspase 3-dependent neurodegeneration in slice preparations, and can be used to test the effects of sustained inflammation on neuronal survival (Alasia et al., 2015; Lossi et al., 2016).
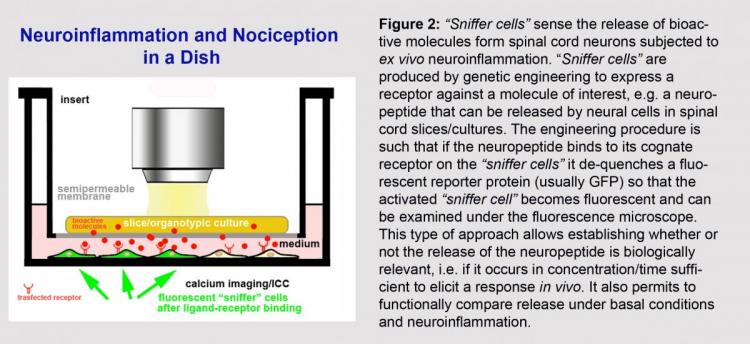
The system is also amenable to pharmacological medium throughput screening as slices can be co-cultured with a biosensor cell line (“sniffer cell”) expressing receptors for an inflammatory factor of interest (Figure 2). This approach is crucial to test the physiological relevance of centrally released mediators of inflammation as it gives cues on their capability to elicit a functionally relevant response in vivo. Neurons derived from human-induced pluripotent stem cells can also be used as biosensor “sniffer” cells to obtain important translational information on the response of human neurons to slice-derived inflammatory mediators (Ghoochani et al., 2016).
References
- Alasia S, Cocito C, Merighi A, et al. (2015). Real time visualization of caspase-3 activation by fluorescence resonance energy transfer (FRET). In: Neuronal Cell Death. Methods in Molecular Biology (Methods and Protocols), vol 1254. (Ed. Lossi L, Merighi A), New York: Humana Press/Springer Science+Business Media. doi:10.1007/978-1-4939-2152-2_8.
- Ghoochani A, Yakubov E, Sehm T, et al. (2016). A versatile ex vivo technique for assaying tumor angiogenesis and microglia in the brain. Oncotarget 7(2): 1838-53. doi:10.18632/oncotarget.6550.
- Jackson SJ, Andrews N, Ball D, et al. (2016). Does age matter? The impact of rodent age on study outcomes. Laboratory Animals 51(2): 160-169. doi:10.1177/0023677216653984.
- Lossi L, Cocito C, Alasia S, et al. (2016). Ex vivo imaging of active caspase 3 by a FRET-based molecular probe demonstrates the cellular dynamics and localization of the protease in cerebellar granule cells and its regulation by the apoptosis-inhibiting protein survivin. Molecular Neurodegeneration 11(1): 1-20. doi:10.1186/s13024-016-0101-8.
- Salio C, Ferrini F, Muthuraju S, et al. (2014). Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (GDNF). J Neurosci 34(41): 13819-33. doi:10.1523/JNEUROSCI.0808-14.2014.
Collaborations are sought with academics and/or pharmaceutical and biotechnology companies to:
- Expand (e.g. by the use of microfluidic culture systems) and validate SCSPs for preclinical drug development and precision medicine.
- Provide a larger panel of candidate pain-controlling drugs/molecules that could be tested and shortlisted for further development using SCSPs.
- Explore the utility of the slice model for investigating disease models, for example using the spinal cord of an EAE mouse to study Multiple Sclerosis.
In the framework of a collaborative project/venture, the researchers offer:
- The possibility to test any compound of interest with the researcher’s SCSPs.
- The development of slice platforms from other areas of CNS (postnatal cerebellar slices are already routinely in use in the lab) for the study of neurodegeneration.
- The development of slice platforms from aged brains.
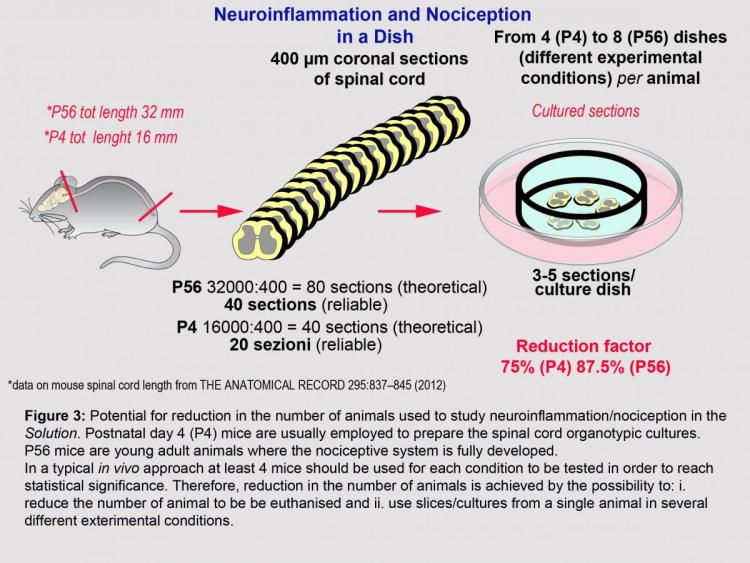
Using SCSPs has the potential to reduce the number of animals used in experiments by enabling multiple slices to be obtained from each animal, therefore decreasing the number of animals required per study. As an example, 20 slices (400 µm thick) can be easily obtained after dissection of the spinal cord from a P4 mouse. Multiple slices can be cultured in each well of a multi-well plate, enabling different experimental/pharmacological challenges to be performed on SCSPs obtained from the same animals (Figure 3). In an in vivo approach where eight mice were used per group, and four groups studied, SCSPs have the potential to reduce the number of mice used from 32 to 8. The technology also provides a novel medium throughput screening platform amenable to preclinical development of pain-targeted therapeutics.
The number of animals required can be further reduced by avoiding the need to specifically generate new transgenic mouse strains. SCSPs can be transiently transfected to express one or more proteins of interest, removing the requirement to breed transgenic animals for some experiments.
For more information: www.morfovet.it/Merighi/Index.html.
Overview | Impact | Publication
Overview
With CRACK IT Solutions funding, Professor Merighi is now working with a team led by Professor Eustace Johnson at the University of Chester, UK. Together, they will use the spinal cord slice platforms model to study the effects of transplanting mesenchymal stem/stromal cells into the spinal cord, to better understand their effects on neuroinflammation, central neuropathic pain and neuronal hyperexcitability. This system has the potential to reduce the number of animals used for these studies by up to 50%, as multiple slices can be cultured from a single animal.
Impact
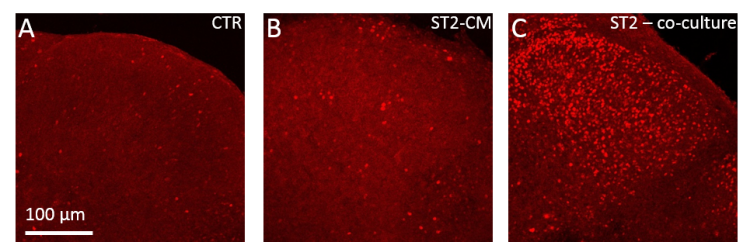
350 µm thick spinal cord slices were taken from CD1 mice (postnatal day seven/eight, both sexes) using a vibratome and co-cultured with ST2 cells (mouse bone marrow-derived stromal cell line) or ST2 cell-conditioned medium (ST2-CM) for 72 hours. The conditioned medium was obtained by culturing ST2 cells in neurobasal medium for three days, after which the medium was harvested. Figure 1 below demonstrates that there is an increase in c-fos expression, a marker of neuronal activation, in the nuclei of the spinal cord slice cells co-cultured with ST2 cells, in comparison to those exposed to ST2-CM only. This indicates that co-culture with ST2 cells may be more effective at reconstructing lost circuits and activating spinal cord neurons than the conditioned medium. However, a direct comparison between co-cultured cells and conditioned medium is technically difficult because the optimal number of ST2 cells transplanted onto the spinal cord slices needs to be established. Therefore, this study continued with ST2-CM only.
Figure 1: c-fos expression in organotypic murine spinal cord cultures in control (A), after 72 hours in ST2-CM (B) or 72 hours co-cultured with ST2 cells (C).
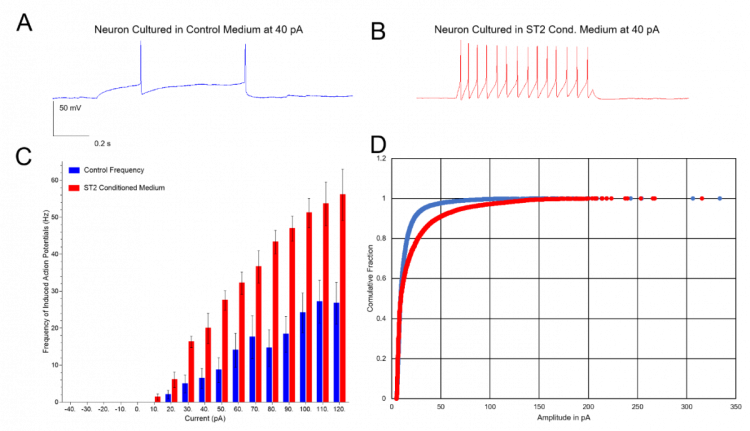
To further investigate neuronal activity, patch clamp experiments were performed on individual neurons in the spinal cord slice after culture with ST2-CM for 72 hours. The results in figure 2 indicate an increase in firing frequency and excitatory post synaptic current amplitude in the spinal cord slice neurons co-cultured with ST2-CM. This data suggest that the ST2-CM promotes neuronal activity in spinal cord slices by preserving and strengthening local synaptic connectivity and transmission.
Figure 2: Patch-clamp recordings from neurons in control medium and ST2-CM. A-B: firing activity induced by 40 pA depolarizing steps in control (blue, n=15) and in ST2-CM (red, n=4). C: Firing frequencies induced by current steps from -40 to 120 pA. Mean frequency: 15.06±4.14 Hz (controls) and 36.57±4.44 Hz (ST2-CM; two-tailed t-test, p=0.0103). D: Cumulative plot of excitatory post synaptic current amplitudes recorded in voltage clamp (holding -70 mV) in control (blue, n=17) or in ST2-CM (red, n=5). ST2-CM induced a right shift in the distribution, indicating an increase in excitatory post synaptic current amplitude (Kolmogorov-Smirnov test, P<0.001).
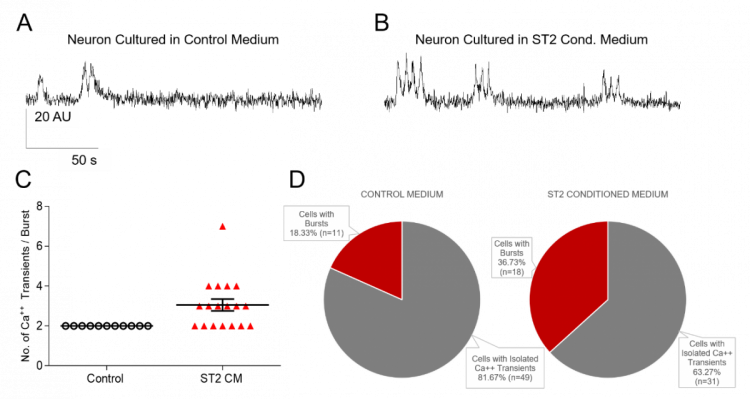
Calcium-imaging experiments were performed on the neurons in the spinal cord slices to analyse a greater number of neurons simultaneously and explore the activity and connectivity of the entire neuronal network. The results demonstrate that both control and ST2-CM treated cultures express spontaneous calcium activity, in the form of isolated calcium transients or burst like activity (figure 3A-B). However, burst activity was significantly heightened in the presence of ST2-CM, suggesting that the pattern of intracellular calcium oscillations is modified by exposure to STC-CM. This again indicates there may be improved connectivity of the neurons, as the network appears to be more active. Further work needs to be done to ascertain which biological mediators are present in the conditioned medium that drive these responses, but these data suggest they are present at active concentrations.
Figure 3: Calcium imaging in control medium and ST2-CM. Recordings of burst-like activity in neurons in control (A) and ST2-CM (B). C: Number of calcium transients per burst in control (n=11) and ST2-CM (n=18) - Mann-Whitney test, p=0.0016. D: Patterns of activities in control and ST2-CM.
Overall, the data indicates that ST2 cells and ST2-CM promote neuronal activity in spinal networks, influencing the rhythmicity of network activity and increasing local synaptic connectivity. ST2-CM is a promising substrate for repair after injury, as a robust synaptic connectivity between neurons is an important factor in tissue repair. Similarly, transplanted ST2 cells appear to be able to activate spinal cord neurons as demonstrated after c-fos immunostaining. The effects of ST2 transplanted cells on neuronal activity is still being investigated to optimise the cell dosage before comparing the results to those obtained with ST2-CM.
The knowledge gained during the project has changed the approach used to study spinal cord biology in the two institutions. The implementation of a co-culture has enabled researchers at the University of Turin to expand their use of spinal cord slices and the collaborator has moved from severe in vivo spinal cord injury models to the ex vivo models described here. Typically, ten to 12 slices can be obtained from each mouse and can be used for either control or treatment groups, significantly reducing the number of animals required. For the experiments in this report eight mouse pups were used, whereas the researchers estimate that the same experiments in vivo, would have required approximately 60 animals (i.e. ten animals as control and ten as treatment for each technical approach).
Publication
Lossi L, Merighi A (2018). The Use of ex Vivo Rodent Platforms in Neuroscience Translational Research With Attention to the 3Rs Philosophy. Front. Vet. Sci. 5:164. doi.org/10.3389/fvets.2018.00164.