Functional optical coherence tomography (OCT) enables high quality, ultrafast, qualitative and quantitative characterisation of tissue structure and function in vivo. It can be used in basic biomedical research and clinical medicine as a rapid non-invasive method for tissue disease diagnosis, long-term tissue monitoring and optimisation of treatment. Animals can be studied longitudinally using this approach, greatly reducing the number of animals required for each study. The technology developers, from the University of Dundee, are seeking partners interested in applying the system to their research to assess the utility of the technology in a wide variety of applications.
Through CRACK IT Solutions, Professor Huang and her team are now working with Thea Pharmaceuticals and Keele University to explore the potential use of OCT in monitoring corneal wound healing. Thea Pharmaceuticals have recently developed a new drug to promote corneal ulcer healing. OCT will be used to monitor the effect of this drug on tissue repair and regeneration in a human corneal tissue model.
Tissue assessment is important in a variety of different applications, including clinical diagnosis and treatment, pharmaceutical and chemical development, and tissue engineering. The most basic and important parameter assessed is tissue structural information, which indicates change on a cellular level. Histological analysis of biopsied tissue remains the gold standard for tissue assessment. It enables clear visualisation of cellular behaviour and structural architecture (Baum CL and Arpey CJ, 2005). However, this method has some limitations: 1) it is invasive; 2) analysis cannot be repeated on the same site; 3) it provides a limited understanding of the changes in the elasticity, microangiography, blood flow velocity and direction. Consequently, a myriad of non-invasive investigative techniques have been developed to aid tissue assessment.
Various tissue imaging modalities such as ultrasound imaging (Kiessling et al., 2014; Mahmoud et al., 2013; Wells et al., 2011) and MRI (Fanea et al., 2012; Mariappan et al., 2010) are available and used for tissue characterisation. However, these techniques are known to lack accuracy in the quantitative information they provide due to low imaging resolution and mechanical sensitivity. Thus they have difficulties in differentiating and identifying early and subtle changes. However, structural information alone is not enough to fully understand the tissue physiology.
Functional imaging technologies for tissue characterisation are being increasingly used in a variety of applications, for example in disease/cancer diagnosis and for real time assessment in tissue engineering. Tissue characterisation includes measurement of microvascular distribution, blood velocity mapping, and tissue stiffness mapping. These act as indicators of functional changes and variations in the micro-structure that are useful for understanding tissue pathophysiology. The vasculature is a key player in promoting tissue survival as it carries the tissue's blood supply and is the site for the initiation of inflammatory events in the affected area. The alteration of mechanical properties of biological tissues, especially the stiffness, directly correlates with the tissues’ pathological status.
References
- Baum CL, Arpey CJ (2005). Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 31(6): 674–86. doi:10.1111/j.1524-4725.2005.31612.
- Fanea L, Fagan AJ (2012). Magnetic resonance imaging techniques in ophthalmology. Molecular Vision 18: 2538-2560.
- Kiessling F, Fokong S, Bzyl J, et al. (2014). Recent advances in molecular, multimodal and theranostic ultrasound imaging. Advanced drug delivery reviews 72: 15-27. doi:10.1016/j.addr.2013.11.013.
- Mahmoud AM, Sandoval C, Teng B (2013). High-resolution vascular tissue characterization in mice using 55 MHz ultrasound hybrid imaging. Ultrasonics 53(3): 727-738. doi:10.1016/j.ultras.2012.10.017.
- Mariappan YK, Glaser KJ, Ehman RL (2010). Magnetic resonance elastography: a review. Clinical anatomy 23(5): 497-511. doi:10.1002/ca.21006.
- Wells PNT, Liang H-D (2011). Medical ultrasound: imaging of soft tissue strain and elasticity. Journal of the Royal Society Interface 8(64): 1521-1549. doi:10.1098/rsif.2011.0054.
OCT is an emerging non-invasive assessment tool that overcomes many weaknesses inherent in other equivalent modalities and is free of side-effects (Tomlins et al., 2005; Welzel, 2001). It is a real-time tomographic imaging technique using low-intensity infrared light focused within living tissue. Interferometric detection of reflected light enables high-resolution, 2- or 3- dimensional, cross-sectional visualisation of tissue morphology analogous to histology.
Functional OCT has been successfully used in a variety of clinical applications such as ophthalmology, dermatology and cardiology (Fanea et al., 2012; Tomlins 2005; Welzel 2001), and has huge potential in pharmaceutical and chemical development and as a tool for fundamental research. The system can provide high-resolution structural imaging as well as functional information on parameters such as stiffness change and microvascular structural change. It also allows for differentiation between small alterations in mechanical properties, for example between benign and early malignant tumours (Li et al., 2015). It has a broad spectrum of applications for serial small animal imaging in areas such as developmental biology, tissue engineering and the development of new biocompatible materials.
This newly developed system combines both structural imaging and characterisation of tissue, making it an important tool for monitoring tissue development and survival with several advantages over alternative systems:
- Measurement of tissue structure can attain axial and transverse resolution of up to ~6 µm and ~12 µm, with an image depth of up to 3 mm. Based on this, functional OCT has the potential to assess structural changes in living tissue at a microscopic level (Tomlins et al., 2005; Welzel 2001; Li et al., 2015).
- Functional OCT is designed for visualising capillary networks and detecting flow direction/velocity changes as well as determining both the vascular and surrounding tissue remodelling and growth (Wang et al., 2010; An et al., 2010; An et al., 2008; Qin et al., 2011; Kennedy et al., 2014). Functional OCT blood vessel mapping is a label-free technique; it uses the intrinsic light scattering from flowing blood cells within patent vessels to produce the imaging contrast. This novel system has an ultra-high sensitivity to the blood flow down to 4 µm/s (Wang et al., 2010; An et al., 2010).
- Functional OCT enables quantitative characterisations of mechanical properties, e.g. elastogram, with ultra-high sensitivity.
References
- An L, Qin J, Wang RK (2010). Ultrahigh sensitive optical microangiography for in vivo imaging of microcirculations within human skin tissue beds. Opt Express 18(8): 8220–8228. doi:10.1364/OE.18.008220.
- An L, Wang RK (2008). In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express 16(15): 11438–11452. doi:10.1364/OE.16.011438.
- Fanea L, Fagan AJ (2012). Magnetic resonance imaging techniques in ophthalmology. Molecular Vision 18: 2538-2560.
- Kennedy BF, Kennedy KM, Sampson DD (2014). A Review of Optical Coherence Elastography: Fundamentals, Techniques and Prospects. IEEE Journal of Selected Topics in Quantum Electronics 20(2): 272-288.
- Li C, Guan G, Ling Y, et al. (2015). Detection and characterisation of biopsy tissue using quantitative optical coherence elastography (OCE) in men with suspected prostate cancer. Cancer Letters 357(1): 121-128. doi:10.1016/j.canlet.2014.11.021.
- Qin J, Jiang J, An L, et al. (2011). In vivo volumetric imaging of microcirculation within human skin under psoriatic conditions using optical microangiography. Lasers in surgery and medicine 43(2): 122–129. doi:10.1002/lsm.20977.
- Tomlins PH, Wang RK (2005). Theory, developments and applications of optical coherence tomography. Journal of Physics D: Applied Physics. 38(15): 2519-2535. doi:10.1088/0022-3727/38/15/002.
- Wang RK, An L, Francis P, et al. (2010). Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Optics letters 35(9): 1467–1469. doi:10.1364/OL.35.001467.
- Welzel J (2001). Optical coherence tomography in dermatology: a review. Skin Res Technol 7(1): 1-9. doi:10.1034/j.1600-0846.2001.007001001.x.
Functional OCT has been designed and developed through a collaboration between medical photonics groups at the University of Dundee and the University of Washington. The researchers focused on state-of-the art, non-invasive and high resolution optical imaging systems for in vivo and real-time characterisation of tissue.
Partners interested in applying this system to their research are sought to assess the utility of the technology in a wide variety of applications. Partners would ideally have access to in vivo animal data and/or clinical models of developing or diseased tissue which is undergoing structural and functional change, e.g. in tissue engineering, diabetes or wound healing programs. Such collaborators could be large companies, biotechs, academics, or healthcare providers.
Input and guidance from potential commercial partners would also be welcomed, to make the system more portable for various environments according to their needs.
Information about IP
The University has a flexible approach to the protection and exploitation of any foreground IP. It would look to derive benefit from the IP but if a partner company was best placed to develop the commercial route then the ownership or an exclusive licence to the technology would be agreed.
Functional OCT can provide high resolution images of tissue in vivo, enabling ‘optical biopsy’ without taking a real piece of tissue. The quality of images from functional OCT can be as high as microscopy images and the tissue assessment can be tracked and studied continuously at a local level. Concrete diagnosis can be made earlier resulting in less suffering and shorter experimental times for the animal. The system also circumvents the need for more invasive tests such as vascular access and stiffness measurement by mechanical load.
Functional OCT enables in vivo study on the same tissue site and thus can allow for a reduction in the number of animals necessary in future studies. This would also reduce the time required to complete the analysis if less samples have to be processed. Moreover, by having the ability to serially sample the same animal, the researcher can track an individual animal over time, allowing more complete data collection. Traditional approaches to generate this data require groups of animals to be sacrificed at multiple time points. The number of animals required is further reduced as the lack of inter-animal variation means that the standard deviation found with this non-invasive method is smaller than that associated with the current methods.
Overview | Impact | Publications
Overview
Through CRACK IT Solutions, Professor Huang and her team are working with Thea Pharmaceuticals and Keele University to explore the potential use of OCT in monitoring corneal wound healing. Multiple animal models for corneal wound healing are currently used including rodent, rabbit and dog models. These can be extremely painful due to the high density of the nerves in the eye and can result in infections. Thea Pharmaceuticals has recently developed a new drug to promote corneal ulcer healing. OCT will be used to monitor the effect of this drug on tissue repair and regeneration in a human corneal tissue model (developed by collaborators at Keele University), providing an example of replacing the use of animal models for assessing drug effects on human tissue analogues.
Impact
A 1 mm thick tissue engineered human corneal model was created with their collaborators at Keele University using human primary keratocytes (P10872 from Innoprot). A lesion was induced in the corneal model (using a biopsy punch), to assess the effect of Cacicol®, a drug provided by Thea Pharmacueticals that promotes corneal ulcer healing. Glass top lids with good light penetration were fabricated, allowing the lids to remain on during OCT to maintain a sterile environment inside the dishes (used for culture and imaging). This enables continuous monitoring of the tissue regeneration before and after the administration of Cacicol®.
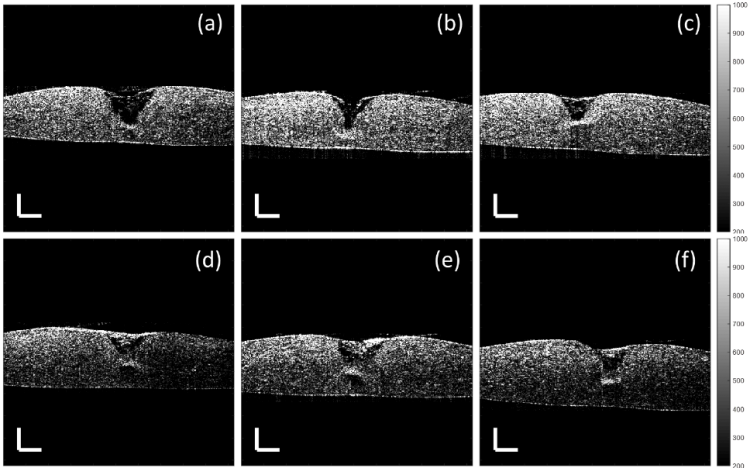
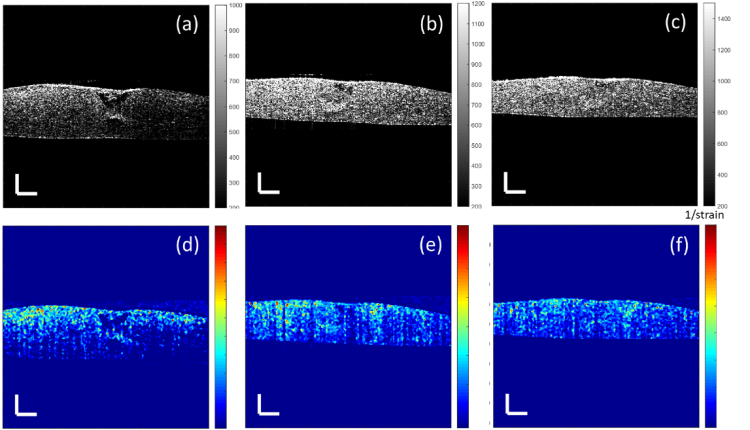
Model thickness and lesion depth were measured every two/ three days for two weeks using OCT with an axial resolution of 8.9 μm and a transverse resolution of 18 μm in air, providing high resolution depth-resolved images. The study demonstrated that lesions treated with 4% Cacicol® exhibited greatest recovery with the largest volume of regenerated tissue (Figure 1e) at day six when compared to the non-treated group (Figure 1a). This suggests that 4% Cacicol® may aid tissue regeneration early in the wound healing process.
Figure 1. Cross-sectional OCT images of the corneal models with a 1.5 mm punched lesion at day six, treated with a) the culture medium only (control), (b) 0.5%, (c) 1%, (d) 2%, (e) 4% and (f) 6% Cacicol® in the same volume of the culture medium. The scale bar is 300 µm.
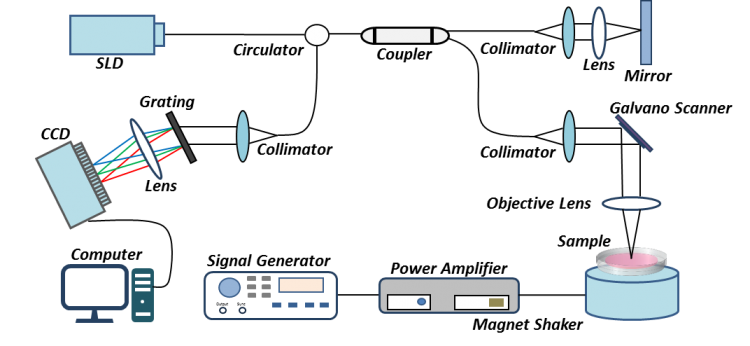
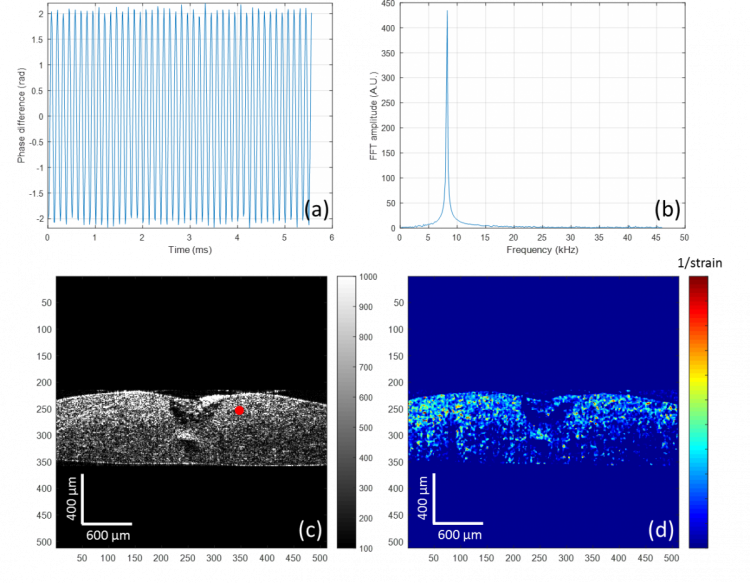
It is also well known that in addition to structural changes, the mechanical properties of the cornea are affected by scar tissue formation and could lead to permanent loss of vision. The OCT system has been modified to non-invasively map the elastic properties of soft tissue based on the mechanical contrast of the tissue, this novel technique is termed optical coherence elastography (OCE). To adapt the OCT system for elastography, a magnet shaker was added to generate vibrations in the axial direction within the sample (Figure 2). The vibration signals are acquired by the OCT using multiple repeated scans taken at the same transverse location over time to construct a cross-sectional image. The elastogram generated is colour coded with red indictating high stiffness and blue representing soft mechanical properties (Figure 3d).
Figure 2. A schematic of the vibration OCE (OCT elastography) system setup for monitoring the corneal wound healing process. SLD (superluminescent diode), CCD (charged coupled device) line-scan camera.
Figure 3. Mechanical evaluation of a corneal model with a 1.5 mm punched lesion, after a week in culture with 4% Cacicol®. (a) Sinusoid vibration signal against time, of a typical point identified by the red dot in (c); (b) Fourier transform of the vibration signal creates a peak amplitude at the modulated frequency, which is related to the displacement and can be used to calculate the strain value; (c) cross-sectional image (produced by OCT) of the corneal model including tissue regeneration; (d) the elastogram of the corneal wound, generated with the reciprocal of the strain value.
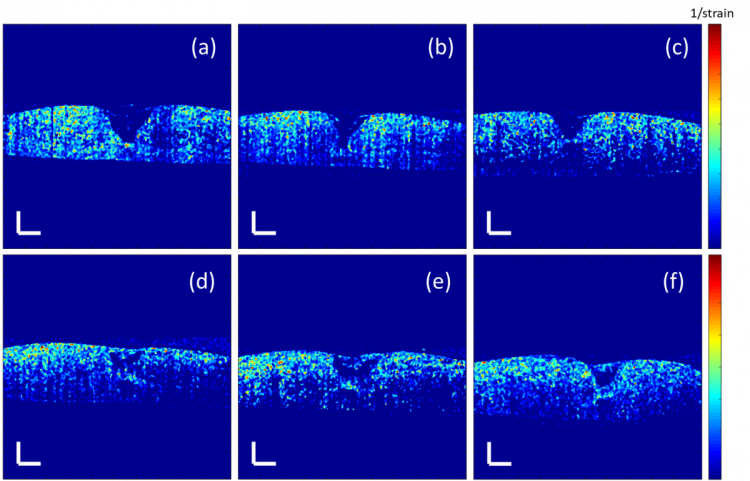
Model thickness and lesion depth were measured every two/ three days for two weeks using OCE. At day six, not all the regenerated tissue in the structural images in Figure 1 was observed in the elastograms in Figure 4. This indicates that some of the regenerated tissue may be too loose to form any alignment of collagen fibres to provide the local mechanical strength and hence the tissue has not fully recovered after a week.
Figure 4. Elastograms of the corneal models with a 1.5 mm lesion at day six treated with a) the culture medium only (control), (b) 0.5%, (c) 1%, (d) 2%, (e) 4% and (f) 6% Cacicol® in the same volume of the culture medium. The scale bar is 300 µm.
Time course studies were carried out to investigate the regeneration of new tissue in the in vitro lesioned corneal models. Figures 5 and 6 illustrate the effect of 4% and 2% Cacicol® on the wound healing process. Although the lesions treated with 4% Cacicol® exhibited the greatest recovery at day six, those treated with 2% Cacicol® presented better recovery at days ten and 13. At the end of the study, the samples were investigated by confocal microscopy (after fixation and histology), which corroborated the structural results obtained using OCT.
Figure 5. Cross-sectional OCT images (a-c) and elastograms (d-f) of a corneal model with a 1.5 mm punched lesion treated with 4% Cacicol® at (a) day 6, (b) day 10, (c) day 13. The scale bar is 300 µm.
Figure 6. Cross-sectional OCT images (a-c) and elastograms (d-f) of a corneal model with a 1.5 mm punched lesion treated with 2% Cacicol® at (a) day 6, (b) day 10, (c) day 13. The scale bar is 300 µm.
These results demonstrate that the OCT/ OCE system is sensitive enough to detect the structural/ mechanical changes of the ulcer tissue during regeneration of the human tissue engineered corneal model. The successful establishment of this in vitro human cornea ulcer model could replace the use of some animal models.
Using animal models to assess the therapeutic effects of new drugs is unreliable due to metabolism differences between animal species and humans, leading to lack of predictability of ocular drug bioavailability in humans. Human tissue-engineered platforms provide a more relevant model to better predict the outcome in humans, whilst also replacing the animal models currently used. For example, this project included five repeats for the control and each dose (0.5%, 1%, 2%, 4%, 6%) of Cacicol® per lesion size (5 sizes ranging from 350 μm to 2 mm), which equates to 150 tissue engineered models that would have otherwise required 75 rabbit or mouse models. The tissue engineered corneas also prevent the significant suffering the animal would have experienced from the chemical or laser lesion of the eye.
The non-invasive OCT/ OCE system enabled the structural and mechanical measurements to be made whilst maintaining the sterile environment of the corneal model, allowing the same sample to be monitored throughout ulcer healing, achieving more reliable results due to the lack of variation between samples. This could lead to a reduction in the number of in vitro models required and associated costs. For example, this study required seven times less in vitro models for the eight timepoints measured, compared to studies where one in vitro model is required for each timepoint. Being able to continuously monitor one sample and provide region resolved measurements of corneal tissues, could increase the uptake of in vitro tissue engineered corneal models, replacing the current animal models used.
The Dundee group is also working with Tissue Repair Technologies, who often use mice for the study of skin ulcers. With the establishment of human tissue engineered skin models, combined with the OCT/ OCE technology, the Dundee group could replace the mouse models currently used to assess drugs for skin ulcers, demonstrating the wider applicability and 3Rs benefits this technology offers.
Publications and posters
Ling Y, Li C, Wang Y, et al. (2017). Characterization of corneal wound healing process using an in-vitro 3D corneal model and optical coherence elastography poster.