ProBE IT
The aim of this Challenge was to develop a sensitive, non-invasive, single imaging modality, through the combination of existing or development of new techniques, to define biodistribution of macromolecules in vivo and enable better predictions of efficacy.
Phase 2 not awarded
Phase 1 awarded
Two Phase 1 Awards were made to project teams led by:
- Dr James McGinty, Imperial College London, £98,439
- Professor Neil Williams, KWS BioTest, £99,938
Challenge launched
Sponsored by GSK, the ProBE IT Challenge aims to develop a sensitive, non-invasive, single imaging modality, through the combination of existing or development of new techniques, to define biodistribution of macromolecules in vivo and enable better predictions of efficacy.
Background
The proportion of new biological drugs in development is continuing to increase due to good probabilities of clinical success (12% for biologics versus 7% for small-molecule drugs), higher peak sales and perceived lack of generic competition. This class of drugs includes macromolecules such as antibodies and oligonucleotides that present unique challenges in candidate drug selection. To improve efficacy, detect toxicity and reduce drug attrition, it is essential to understand the biodistribution properties of these macromolecules early in development to ensure they are being delivered to the intended site of action. Linking biodistribution to disease progression through the use of efficacy biomarkers also provides greater confidence in predicting the ultimate success of the molecule.
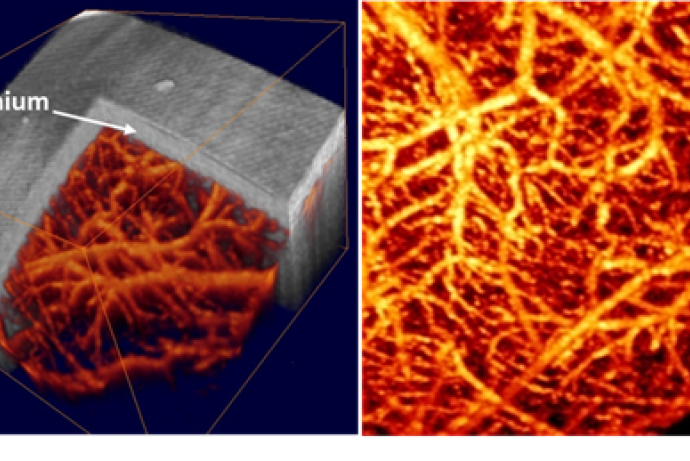
PET imaging can be used to determine biodistribution information for drug candidates and has clear advantages when looking at small molecules where a single atom can be exchanged for a radioactive ligand without changing the intrinsic properties of the molecule. Currently, there are no routinely available and accessible techniques to allow whole body distribution of larger macromolecules to be measured over a longitudinal timeframe of several days within a single animal. Traditionally, animals are dosed with the drug candidate and biodistribution is subsequently assessed using ex vivo techniques which have a considerable impact on the total number of animals required to gain accurate information on a time course. There are scientific and practical limitations associated with current assessments including the number of animals required, the difficulty in obtaining precise profiles of biodistribution patterns within an animal, the inability to visualise biodistribution in 3D and the need to remove and analyse each individual organ to obtain a profile.
The aim of this Challenge is to develop a sensitive, non-invasive imaging technology to accurately detect and quantify a range of macromolecules in a variety of tissues and link this information to efficacy. If in vivo efficacy imaging biomarkers could be linked to understanding biodistribution within the same animal a powerful paradigm would be realised for biological drug development using fewer animals. Emerging technologies using optical probes in the near infra red (NIR) range such as FRET, which can give information on distribution and potentially conjugation to a receptor, as well as FLIM which provides information on the local chemical environment of the molecule, are potentially of use to this Challenge. However, these are not currently adequate to link biodistribution to efficacy.
3Rs benefits
- The development of imaging tools for routine biodistribution studies would reduce animal use by at least 85%. This could be reduced even further if biodistribution can be linked with non-invasive efficacy readouts within the same animal.
- Linking biodistribution to efficacy will enable more precise measurements to be made allowing earlier stages of disease to be detected that better reflect the clinical situation and reducing the severity of the animal studies (refinement).
- The impact could potentially span many therapeutic areas including oncology, neurodegenerative diseases and diseases that affect the kidney, lung, liver and heart where targeted therapy is the main goal. Earlier efficacy or safety observations, together with biodistribution profiles, may also provide earlier decision points on whether to progress compounds therefore reducing the length of these studies.
- Decisions on whether a macromolecule should be progressed will also be made earlier, resulting in fewer candidate drugs that may be dropped later in development entering regulatory safety and toxicity studies. This is particularly important for antibodies, where often the only relevant species for toxicology studies is the non-human primate.
Phase 1 winners
Project teams led by:
- Dr James McGinty, Imperial College London, £98,439.
- Professor Neil Williams, KWS BioTest, £99,938.
Phase 2
Not awarded