The number of transgenic mice has risen in recent years to the extent that nearly half of all animals in research are now those in transgenic mouse breeding programmes. Ostara is addressing this issue by developing a vaginal pessary designed to efficiently induce pseudopregnancy in female mice. This technology can replace the need for vasectomised males and reduce the number of females required to ensure the timely generation of sufficient pseudopregnant recipients. Ostara seeks collaborators that routinely induce pseudopregnancy using vasectomised males and are also skilled in embryo transfer, to help compare the efficiency of Ostara pessaries with the use of vasectomised males.
Through CRACK IT Solutions Ostara have partnered Cancer Research UK (CRUKs) London Research Institute. This CRACK IT Solutions funded collaboration enabled the evaluation and further refinement of their prototype vaginal pessary system.
Genetically modified mice are widely used in the development of new treatments for, and to better understand the pathophysiology of, an extensive array of diseases and conditions, ranging from cancer and heart disease through to autoimmune disorders and pregnancy loss. In order to produce such mouse models, genetically manipulated embryos are transferred to recipient mothers to produce litters of transgenic founders from which transgenic lines can be bred. Furthermore, transgenic embryos are often frozen for rederivation of important lines at a later date.
Current practice requires the induction of pseudopregnancy in prospective recipients, thereby enabling them to become hormonally and physiologically competent to enable the implantation of transferred embryos. Pseudopregnancy is generally achieved by mating prospective recipients with sterile males which are frequently surgically vasectomised for this purpose. This is an inefficient process and requires large numbers of male and female mice. Strategies to avoid the need to perform vasectomies by using mutant mice (e.g. T145H-Re) with impaired spermatogenesis have been developed (Lyon MF, Meredith R 1966; Lyon MF, et al. 1996), however the production of sterile males is inefficient (circa 25%) and uses many mice.
References
- Lyon MF, Meredith R (1966). Autosomal translocations causing male sterility and viable aneuploidy in the mouse. Cytogenetics 5: 335-354.
- Lyon MF, Rastan S, Brown SDM (1996). Genetic variants and strains of the laboratory mouse. 2 Volumes, Oxford, New York, Tokyo: Oxford University Press.
Ostara Biomedical is a small UK company focused on developing a product designed to streamline procedures and reduce the number of animals used in the production of transgenic mice. Ostara is developing a novel vaginal pessary that can be rapidly administered to female mice by animal technicians and totally replaces the need for vasectomised (or otherwise sterile) males in transgenic breeding programmes. Use of the pessary system is also more efficient at inducing pseudopregnancy than mating with sterile males and Ostara aims to reduce the number of females in the pools maintained by users by an average of 50%, whilst ensuring the timely production of adequate numbers of pseudopregnant recipients for embryo transfer.
Transgenic embryos are commonly generated by microinjecting DNA constructs into zygote pronuclei, in which case zygotes are typically transferred into pseudopregnant recipients at 0.5 days post coitus. Ostara has demonstrated proof of concept by obtaining successful pregnancies after transfer of such early stage embryos, using its pessary-based system to induce pseudopregnancy. However, given that transgenic mice are also created through the injection of transfected embryonic stem cells into blastocysts, prior to transfer into pseudopregnant recipients at 2.5 days post coitus, Ostara now seeks to identify and work with partner(s) skilled in the micromanipulation of such later stage embryos.
Ostara’s Solution will have an immediate impact on streamlining procedures and reducing the number of animals and production costs associated with transgenic breeding programmes. Ostara would like to evaluate pregnancy success rates following transfer of both normal and transgenic zygotes and blastocysts into pseudopregnant females that have been induced using the pessary. In addition, the successful pregnancy rate following transfer of embryos that have been stored frozen, will be ascertained. The aim is to demonstrate efficacy of the Ostara pessary in supporting the establishment of successful pregnancies following transfer of both early and later stage embryos that have been manipulated and stored in accordance with commonly used procedures. Ostara aims to refine its product and launch this on the market by 2015.
Ostara is working with expert partners (in both commercial and academic sectors) to develop its proprietary technologies and currently seeks a collaborator(s) that is both routinely inducing pseudopregnancy using vasectomised males and is also skilled in embryo transfer, at both the zygote and blastocyst stage. The ideal partner will also have experience in rederivation of frozen embryo stock. Ostara anticipates working with partners to devise a detailed experimental programme to compare the efficiency of Ostara pessaries with the use of vasectomised males.
The demand for this solution is highlighted by the steady, long-term increase in the numbers of mice used in transgenic breeding procedures. The UK Home Office statistics indicate a 22% increase (+363,100) in the breeding of genetically modified animals, mainly mice, (to 1.98m procedures) in 2012 alone (Home office, UK government website). The Ostara pessary will reduce some of this use in the following ways:
- Replacement : The Ostara pessary will replace the current requirement for vasectomised males, which currently undergo invasive surgical procedures and are usually kept for six months only to avoid additional inefficiencies introduced by decrease in potency as they age.
- Reduction: Use of the pessary will enable breeders to reduce the size of the pool of females used as recipients, as pseudopregnancy can be induced in a reliable and timely manner, within the optimal time window for embryo transfer, when recipients are at peak age and weight to support successful pregnancy.
Reference
Overview
Ostara Biomedical have worked extensively with partners to develop their proprietary technology, but now need to validate the approach with collaborators routinely inducing pseudopregnancy using vasectomised males. Through the CRACK IT Solutions technology partnering scheme they have identified multiple potential partners but have selected to work with the Cancer Research UK (CRUKs) London Research Institute. This CRACK IT Solutions funded collaboration will allow Ostara Biomedical to capitalise on CRUKs expertise and excellent animal facilities to support the evaluation and further refinement of their prototype vaginal pessary system.
Impact
This study aimed to validate the Ostara Biomedical vaginal pessary system as a tool to (i) replace the use of vasectomised/sterile males in pseudopregnancy induction (essential for embryo implantation) and (ii) to further enhance embryo implantation rates, in mice.
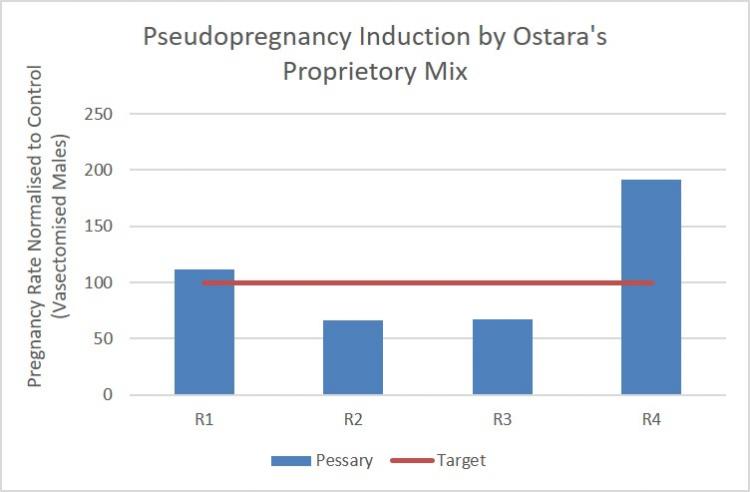
During early testing of the pessaries it became evident that further optimisation of the pessary manufacture and formulation would be necessary to preserve the activity of the active ingredients. A new partnership was established with ThioMatrix to complete this aspect of the project. In parallel, in vivo formulation testing was carried out in collaboration with CRUK's London Research Institute and additional animal units to explore the efficacy of three different active ingredient combinations. The results demonstrated that alternative combinations of additional active ingredients yielded similar or enhanced rates of pseudopregnancy induction in comparison to the original formula (Figure 1), increasing the number of active ingredients available for formulation. This is critical to de-risking future product development and bringing it closer to commercialisation.
Figure 1: Pseudopregnancy induction by Ostara’ proprietary mix of active ingredients. R1 (n=14 pessary, n=13 control) is Ostara’s original recipe, R2 (n=28 pessary, n=27 control) and R3 (n=18 pessary, n=18 control) are revised recipes yielding only 65% of the control (vasectomised males) success rate. R4 (n=18 pessary, n=18 control) is a revised recipe that exceeded the vasectomised male pregnancy rate.
Working with Marble Product Design, Ostara Biomedical has also developed an ergonomic, safe and reliable delivery mechanism for the vaginal formulation (Figure 2). Although not directly funded by the NC3Rs, CRACK IT Solutions funding allowed Ostara Biomedical to utilise other resources to develop the applicator in parallel to the formulation, significantly shortening the product development time. This has received positive user feedback from Ostara Biomedical’s partner unit at the University of Leeds, particularly in terms of ease of use. Work is ongoing to quantitatively assess the impact on the welfare of the animals in comparison to the traditional use of vasectomised males; however initial screening (based on animal behaviour and visual vaginal tissue observations by unit staff) suggests that there are no adverse effects.
Figure 2: Ostara Biomedical’s proprietary device for vaginal delivery of the formulation. The outer body contains a flexible air bladder which is depressed by the user, delivering a metered dose of the formulation into the mouse vagina.
Overall this product development study has improved manufacturing processes and increased the stability, reproducibility and efficacy of the formulation, leading to the progression of the product towards market. Commercialisation of Ostara Biomedical’s vaginal delivery-based innovation could significantly impact on the use of animals in breeding programmes and research. Across the EU, approximately 1.9 million mice per year are used in the production of pseudopregnant mice in preparation for embryo transfer (Seventh Report from the Commission to the Council and the European Parliament on the Statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union, COM(2013)859). Ostara estimate that the vaginal formulation could reduce the number of mice in pseudopregnancy generation by 50-70% through the replacement of males and improvements in pseudopregnancy induction in females. This estimation would result in approximately 950,000 fewer mice being used in pseudopregnant production across the EU per year. An additional benefit will be that males will no longer need to be surgically sterilised, impacting on the severity level of the production of pseudopregnants for research. This product will be of interest to animal breeders and small research units alike.
The NC3Rs CRACK IT Solutions funding has not only enabled the validation and development of Ostara Biomedical’s vaginal delivery system, but it has also been instrumental in securing additional funding to continue this work stream and engage with potential customers in Europe and USA.
Quote from Ostara Biomedical:
“The CRACK IT Solutions award has been instrumental in enabling Ostara to develop a pseudopregnancy induction alternative to vasectomised/sterile males, thereby effectively replacing the numbers of animals used to this end. In particular, the flexibility of the award has allowed Ostara to progress the product in directions not envisaged at the start of the project, thus bringing the product closer to market. The relationship between Ostara and its Solutions partner CRUK, generated through CRACK IT Solutions funding, has facilitated and provided further collaboration opportunities.”
To find out more about Ostara Biomedical’s vaginal pessary system visit Ostara's website.