RaTS
The aim of this Challenge was to develop and validate a handheld device for objective monitoring of rheumatoid arthritis (RA) progression in conscious rodents (either restrained or unrestrained). While it is expected that Raman transmission spectroscopy will be the technology needed to solve the Challenge, applications involving other approaches (such as Surface-enhanced Raman spectroscopy) are also welcome, provided they do not require the use of transgenic animals, do not substantially complicate the study design or create more burden on the experimental animals (e.g. multiple injections of dyes or nanoparticles).
Publication
Walther AR et al. (2023). In Vivo Longitudinal Monitoring of Disease Progression in Inflammatory Arthritis Animal Models Using Raman Spectroscopy. Analytical Chemistry. 95(7): 3720-3728. doi: 10.1021/acs.analchem.2c04743.
Publication
Stepula E et al. (2024) Label-free 3D molecular imaging of living tissues using Raman spectral projection tomography. Nature Communications. 15(1):7717. 10.1038/s41467-024-51616-y
Phase 2 awarded
A team led by Associate Professor Martin Hedegaard, University of South Denmark, has been awarded £748,697 to deliver Phase 2.
Phase 1 awarded
Three Phase 1 Awards were made to project teams led by:
- Dr Martin Hedegaard, University of Southern Denmark, £98,544.
- Dr Mithun Kuniyil Ajith Singh, Cyberdyne Europe GmbH, Germany, £99,360.
- Professor Jonathan Cooper, University of Glasgow, £99,541.
Challenge launched
Sponsored by GlaxoSmithKline and Galvani Bioelectronics, the RaTS Challenge aims to develop and validate a handheld device for objective monitoring of rheumatoid arthritis (RA) progression in conscious rodents (either restrained or unrestrained). While it is expected that Raman transmission spectroscopy will be the technology needed to solve the Challenge, applications involving other approaches (such as Surface-enhanced Raman spectroscopy) are also welcome, provided they do not require the use of transgenic animals, do not substantially complicate the study design or create more burden on the experimental animals (e.g. multiple injections of dyes or nanoparticles).
Background
RA is a chronic inflammatory autoimmune disorder characterised by persistent joint inflammation which leads to cartilage and bone damage (Picerno et al., 2015). Over 400,000 people in the UK have RA (Symmons et al., 2002) and the associated cost to the UK economy alone is estimated to be £3.8-4.8 billion per year (National Collaborating Centre for Chronic Conditions, 2009). Various pharmacological treatments are being developed to alleviate symptoms and slow disease progression with early, aggressive intervention yielding the best results.
One of the bigest challenges in the study of RA both in humans and animal models is the imaging of the affected joints. Several different imaging approaches are used, each with benefits and limitations.
Indirect in vivo measurements
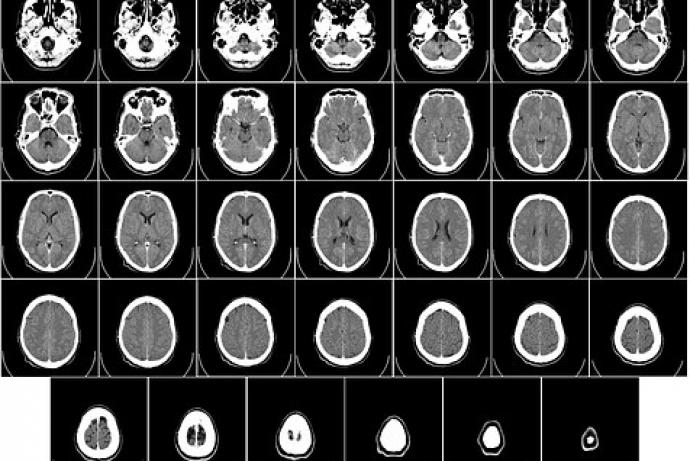
In animal models, the induced joint pain and inflammation in concious rodents can only be monitored indirectly by detecting paw withdrawal in response to noxious or mechanical stimuli and measurements of joint swelling using callipers or a plethysmometer. Under terminal anaesthesia, more direct measurements are possible, such as drawing synovial fluid from the inflamed joints for biochemical analysis and in vivo imaging such as Computed Tomography (CT) or Magnetic Resonance Imaging (MRI).
Direct ex vivo measurements
More detailed measurements of joint damage are currently performed ex vivo:
- Infrared vibrational spectroscopy permits assessment of the loss of structural proteins in cartilage (Croxford et al., 2013) and the loss of minerals in the cartilage and bone matrix (Lim et al., 2011). Two types of infrared vibrational spectroscopy have been applied to ex vivo joints: absorption and Raman spectroscopy. Raman spectroscopy has the advantage over the absorption spectroscopy in that it can detect a wider spectrum of biomolecular changes associated with early cartilage degradation (Lim et al., 2011) and can be used in live animals (de Souza et al., 2014).
- Ultrasound, X-ray/CT, MRI/MR spectroscopy and optical coherence tomography, have all shown promising results in calculating volumes of inflammation and bone/cartilage deformation and demineralisation, with some limited insights of the chemical environments. However, they have low sensitivity and specificity in detecting biochemical changes in the inflammed joint, as well as problems with image resolution, high costs, requirements for specialised facilities, low throughput, use of anaesthetics, and complex image analysis to extract arthritis-specific information (Kirwan, 1999; Marenzana, 2015).
- Fluorescence imaging in vivo is useful to identify some molecular biomarkers; however, these biomarkers are typically few and limited, and have no specificity to the early cartilage destruction. The technique also requires multiple injections of the fluorescent dyes or quantum dots/rods either systemically or intra-articularly; resulting in heterogeneous tissue distribution (Chen et al., 2014) and clearance (Yong et al., 2009; Liu et al., 2013). The variability observed in the pharmacokinetics hinders the longitudinal comparison of measurements taken within individual animals.
- Surface-enhanced Raman spectroscopy has a similar limitation to fluorescence imaging, as it requires multiple injections of the metal nanoparticles in the joints of interest. Furthermore, addition of the biocompatible coatings (such as silica or polyethylene glycol) reduces the efficacy of surface plasmon effect (Andreou et al., 2015). Despite these limitations, the metal nanoparticles exhibit good long-term stability during three week in vivo measurements (Dinish et al., 2015) and its higher signal to noise ratio provides a promising alternative to Raman spectroscopy.
The use of Raman transmission spectroscopy for in vivo monitoring of RA progression in rodents is currently limited by:
- Selection of the optimal infrared laser wavelength for minimal amount of absorption in the skin and bone.
- Optimisation of the optics for maximal amount of light capture by the spectrometer.
- Advanced analysis of the spectral data using adaptive algorithms.
(1) and (2) can potentially be resolved using advances in lasers and optical detectors. While most of the existing Raman lasers operate in the 700-800 nm wavelength range, minimal amount of absorption in the skin tissue requires the use of lasers in the 1000-1100 nm range (Bashkatov et al., 2015). Recent advances in optics using no-slit and multi-slit spectroscopy provide a considerable increase in light capture (Maher et al., 2014; Barnett, 2016; Gooding et al., 2016).
Improved Raman spectroscopy developed through this CRACK IT Challenge could provide richer data regarding the RA disease progression (such as the hypoxia, cartilage erosion, and bone erosion) as compared to the present X-ray/micro-CT approach that evaluates only the bone erosion evident in the later stages of untreated RA, while the onset of cartilage damage appears in the early stages of disease. This could provide a more sensitive metric for evaluating the therapeutic treatments and provide more relevant data to clinical manifestations of the disease.
3Rs benefits
Induction of RA in animal models causes joint swelling and pain which, although closely monitored and countered by the administration of analgesics, can cause significant pain and distress (Hawkins et al., 2015), Currently, the collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AIA) are the most widely used rodent arthritis models in research in academic and industrial laboratories. The CIA model is induced by immunisation with type II collagen (prevalent in articular cartilage), causing inflammation of the joints. The pathogenesis observed in rat CIA makes it more clinically relevant when compared to the AIA model.
GSK and Galvani Bioelectronics have experience of conducting efficacy studies to evaluate the effect of potential therapies in the rat CIA model, typically performing research on over 300 CIA rodents a year. With over ten of the world's major pharmaceutical companies having active RA programmes (GlobalData 2017) and a large academic research sector, application of the technology developed through this Challenge could have significant impact on the numbers and welfare of animal models used.
For X-ray and micro-CT evaluation of the bone erosion, animals are evaluated ex vivo at 21 days post CIA induction, as the bone changes are not evident at earlier timepoints. Successful delivery of this Challenge would allow the detection of the cartilage and bone damage at earlier timepoints (e.g. at seven and ten days post CIA induction) and subsequently at an additional two to three timepoints during administration of anti-arthritic therapy (e.g. at 14, 21 and 28 days). Such improved sensitivity would allow, for the first time, the longitudinal monitoring of a time course of the cartilage and bone healing during the administration of anti-RA treatments. This would also permit within animal assessment which will allow the application of advanced mixed-effects population analyses to further reduce sample size per group.
In summary, Raman compared to X-ray/micro-CT methods will offer these 3Rs benefits:
- Reduced animal numbers (by up to a factor of ten) due to longitudinal measurements and within-animal repeated-measure design.
- Refinement of the animal studies based on shorter study duration and reduced severity of end points (especially shortening the late disease duration) due to higher sensitivity of the cartilage erosion metric during early RA progression and recovery, as compared to the currect ability to detect the bone damage no earlier than day 21.
- More data-rich information generated regarding the cartilage and bone damage and healing from each animal, further reducing the numbers needed.
Full Challenge information
Phase 1 winners
Project teams led by:
- Professor Martin Hedegaard, University of Southern Denmark, £98,544.
- Dr Mithun Kuniyil Ajith Singh, Cyberdyne Europe GmbH, Germany, £99,360.
- Professor Jonathan Cooper, University of Glasgow, £99,541.
Assesment information
Review and Challenge Panel membership
A team led by Associate Professor Martin Hedegaard, University of Southern Denmark and Reader Dr Mads. S Bergholt, Kings College London has developed Raman spectroscopy technology for non-invasive and longitudinal monitoring of disease progression in rodent models of rheumatoid arthritis (RA) to reduce and refine animal use and improve the evaluation of therapeutic treatments in preclinical development.
Symptoms of RA include joint swelling, damage to bone and cartilage and pain. Rodent models used to assess the efficacy of new treatments are often evaluated at late-stage time points, as detecting and monitoring progression of the disease can be challenging in vivo. Techniques used to analyse the effect of treatments for arthritis in vivo can be invasive and time-consuming. Current ex vivo imaging approaches are limited by variability and low sensitivity.
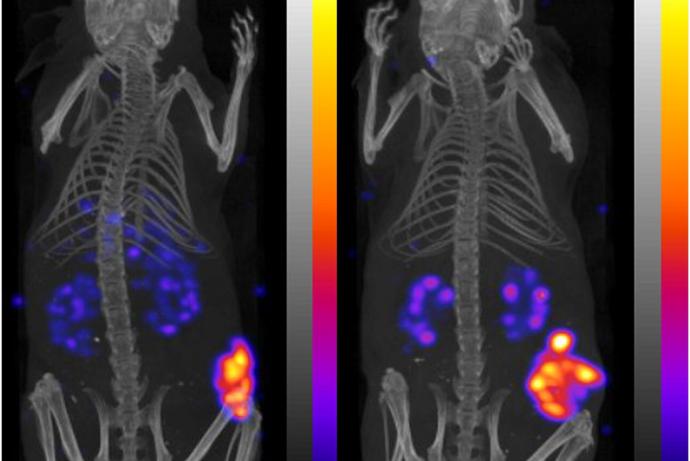
The Raman spectroscopy technology developed to address the RaTS Challenge allows longitudinal measurements of tissue, which could reduce the number of animals required per study through a repeated-measure design. A pilot study assessing changes in the tissue composition of mice with and without arthritis showed that findings from spectroscopy correlated with histological analysis of disease progression over time [1]. The spectroscopy technology may also enable the detection of changes to the bones and surrounding tissue at earlier timepoints, refining the use of rodent models through shorter study durations and reduced severity of endpoints.
The developed technology includes a non-invasive, label-free optical system, Raman Spectral Projection Tomography (RSPT), which monitors tissue alterations at the biomolecular level. This system enables three-dimensional imaging and visualisation of transparent and semi-transparent tissues. By detecting spatial heterogeneities within live 3D tissue constructs in the extracellular matrix, RSPT demonstrates significant potential for non-invasive molecular analysis of living tissues [2].
The collaboration between the University of Southern Denmark and King’s College London was supported by Challenge Sponsors Galvani Bioelectronics and GSK, who provided expertise, guidance and end-user input including insight into use of animal models in RA drug discovery pipelines. Throughout the project, the team disseminated the technology at conferences and meetings across multiple sectors, including Raman spectroscopy and photonics.
To learn more about the technology and how to access it, contact marhe@igt.sdu.dk or mads.bergholt@kcl.ac.uk.
References
[1] Walther AR et al. (2023) In Vivo Longitudinal Monitoring of Disease Progression in Inflammatory Arthritis Animal Models Using Raman Spectroscopy. Analytical Chemistry 95(7) 3720–3728 doi: 10.1021/acs.analchem.2c04743
[2] Stepula E et al. (2024) Label-free 3D molecular imaging of living tissues using Raman spectral projection tomography. Nat Commun 15(7717) doi: org/10.1038/s41467-024-51616-y