Tat-Fit
This Challenge aimed to develop a method that allows tamoxifen to be added to rodent chow without changing its palatability or consumption by the animals. The method must not alter the nutritional value of the food and the final product must be affordable and permit the addition of tamoxifen to the food by researchers and animal care staff.
A team led by Dr Fang Liu, Fluid Pharma Ltd, addressed the Tat-Fit Challenge by developing tamoxifen micropellets encapsulated by a taste-masking coating that are compatible with common rodent feed. The taste-masked product developed by Fluid Pharma means that tamoxifen can be consumed voluntarily by the rodents, avoiding repeated handling that can be required for current methods and improving their welfare.
Challenges briefing webinar
View the Challenges briefing webinar recording to find out more about this Challenge.
Business Growth Scheme awarded
The team at Fluid Pharma Ltd. have been awarded £50k through the Business Growth Scheme (BGS) to support the business and commercial opportunities from technologies arising from the Tat-Fit Challenge.
Challenge completed
Through the Tat-Fit Challenge, the team at Fluid Pharma Ltd have developed tamoxifen micropellets encapsulated by a taste-masking coating that are compatible with common rodent feed.
Challenge awarded
A team led by Dr Fang Liu from Fluid Pharma Ltd has been awarded £96,508 to deliver the project: tamoxifen particles to improve palatability of rodent chow.
Challenge launched
Sponsored by the Mary Lyon Centre this Challenge aims to develop a method that allows tamoxifen to be added to rodent chow without changing its palatability or consumption by the animals. The method must not alter the nutritional value of the food and the final product must be affordable and permit the addition of tamoxifen to the food by researchers and animal care staff.
Background and 3Rs benefits
Conditional inducible transgenesis is widely used in mice to temporally and spatially express or delete genes using CRE recombinase (CRE-ER) (Kim et al., 2018). Induced by the application of the drug tamoxifen, CRE-ER proteins translocate into the nucleus of cells expressing them, leading to recombination at specific Lox P sites flanking the fragment of DNA to be excised.
The CRE system has been widely used to study the function of genes either at specific life stages or in specific tissue types at controlled time points. Although this targeted and inducible transgenesis can help to avoid the lethality caused by some global knockouts where the gene of interest is deleted in every cell in the mouse, the use of tamoxifen can be problematic.
Rodent diet containing tamoxifen is commercially available. The bitter taste of tamoxifen however means that it is not readily consumed by the mice and this can lead to weight loss. Unpalatability can also cause variability in the experimental cohort and confounds findings with the animal ingesting varying amounts of the food and hence tamoxifen. As a result, experiments usually require direct administration of tamoxifen by oral gavage which requires repeated handling and scruffing of the mice and insertion of a gavage cannula, with dosing typically for seven days. This can be highly time consuming for staff. Some facilities administer the compound either intraperitoneally or subcutaneously and this can lead to inflammation at the injection site as tamoxifen is hydrophobic and must be prepared in oil, typically corn oil. For studies involving pregnant mice, dosing particularly by the intraperitoneal route can result in spontaneous abortions.
Tamoxifen preparations can be variable depending on the source and approximately 10% of batches (data from the Mary Lyon Centre) have an adverse effect that results in the animals being removed from the study. The number of animals used is influenced by the challenges associated with effectively administering tamoxifen and the genotype required for the experiment. Some experiments use 192 mice with around 500 mice used in the associated breeding. Improved dosing could minimise the overall number of mice used.
This Challenge aims to deliver a palatable form of tamoxifen that can be prepared within a facility, using the required feed for the experiment. Providing food which contains tamoxifen but is otherwise indistinguishable for the animal will ensure that normal intake is maintained, reducing potential welfare concerns from lack of food and reducing dose variability. This will lead to more consistent recombinase activity, decreasing the variability of the phenotyping data yielded from such experiments, and resulting in a reduction in the number of animals used.
Other benefits
Many drugs are unpalatable, hence the widespread use of tablets in human medicine. It is not possible to dose small rodents with capsules or tablets, therefore any developed method which could potentially be applied in any facility and to any drug would be a significant advantage to the fields of preclinical medicine as well as discovery science (Turner et al., 2011).
Full Challenge information
Assessment information
Review and Challenge Panel membership
| Name | Institution |
|---|---|
| Dr Malcolm Skingle (Chair) | GSK |
| Dr Sara Wells (Sponsor) | MRC Harwell - Mary Lyon Centre |
| Dr Michelle Stewart (Sponsor) | MRC Harwell - Mary Lyon Centre |
| Professor Khuloud Al-Jamal | KCL |
| Professor Joshua Boateng | University of Greenwich |
| Mr James Bussell | University of Oxford |
| Dr Sue Dunkerton | KTN |
| Professor Alexandar Mullen | University of Strathclyde |
| Professor Michael Stocks | University of Nottingham |
The team at Fluid Pharma Ltd, led by Dr Fang Liu, have developed tamoxifen micropellets encapsulated by a taste-masking coating that are compatible with common rodent feed, with support from The Mary Lyon Centre at MRC Harwell.
Tamoxifen is widely used in transgenic research in mice to induce Cre recombinase activity and achieve conditional gene knockouts. Usually, methods of tamoxifen administration include oral gavage and intraperitoneal injection which can be associated with pain, causing stress and complications due to the invasive nature of the delivery method. Fluid Pharma have overcome these issues by creating a non-invasive delivery system for tamoxifen dosing. The team have utilised a unique spray technology that layers tamoxifen onto a microsphere before applying a taste-masking coating using their MicroCoatTM technology. These micropellets are then mixed with food so that tamoxifen is delivered in the diet at normal dosing levels. Unlike other rodent diets that contain tamoxifen and are unpalatable, taste-masking facilitates normal uptake of food, leading to maintenance of a healthy body weigh in mice and significant improvements to tamoxifen dosing consistency.
As there are no handling requirements, the rodents are less likely to experience stress, leading to an improvement in animal welfare. Administering tamoxifen in this way may lead to fewer animals being removed from experiments and reduced data variability due to the reduction in stress and more consistent dosing of tamoxifen. Using Tat-Fit tamoxifen micropellets may also reduce the number of mice needed per experiment as it does not need to account for these losses.
In addition to the welfare and scientific benefits, the coating provides increased protection from exposure to tamoxifen for the researchers conducting experiments that require tamoxifen preparations.
The Sponsors, The Mary Lyon Centre at MRC Harwell, worked with the team to plan the technical aspects of the Challenge and test the coated micropellets in vivo. The team and Sponsors continue to work together to further validate taste-masked tamoxifen technology.
For more information about Fluid Pharma Ltd please visit their website.
Tamoxifen micropellets encapsulated by a taste-masking coating
Fluid Pharma Ltd have developed a tasteless tamoxifen formulation to improve tamoxifen administration to rodents for cre-recombination studies. Micropellets are constructed by layering tamoxifen and a taste-masking coating using their patented MicroCoat™ technology.
Current administration methods for tamoxifen include oral gavage or peritoneal injection, which are invasive, or using tamoxifen-diets, which can cause feed aversion due to the bitter taste of tamoxifen. Both approaches can lead to mortality either due to trauma or weight loss during the dosing period.
Fluid Pharma’s tasteless tamoxifen has been shown have comparable levels of cre-recombination efficiency to other current methods, such as oral gavage, when mixed with powdered feed for oral consumption, demonstrating effective tamoxifen taste masking.
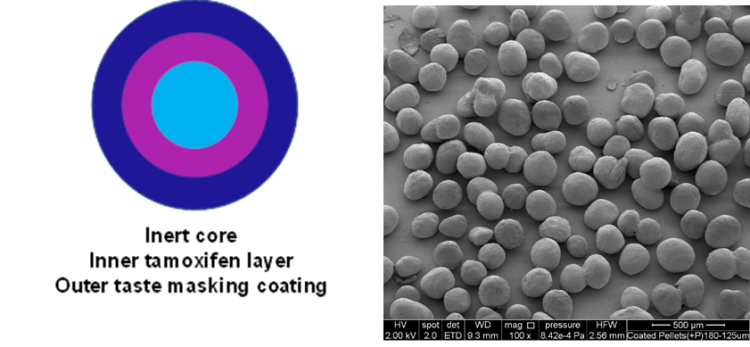
Schematic of the product design (left) and scanning electron microscopy of coated micropellets (right).
The taste-masked product developed by Fluid Pharma means that tamoxifen can be consumed voluntarily by the rodents, avoiding repeated handling that can be required for current methods and improving their welfare. In addition, the coating technology implemented by Fluid Pharma provides a physical barrier between tamoxifen and the product handler, improving safety.
Access the technology
For more information on the taste masked tamoxifen micropellets and how to access the technology, please visit the Fluid Pharma website or contact Fluid Pharma directly.