Researchers at the University of Birmingham have developed a low cost, ex vivo porcine corneal model to rapidly assess ocular drug delivery. The assay uses tissue that is surplus to the food industry and readily available, replacing the in vivo models and better predicting the outcome in the human eye, in comparison to rabbit and rodent models. Industrial collaborators able to provide different classes of ocular drugs and drug penetration data are sought to further validate the assay.
One of the greatest obstructions to topically applied drugs in the eye is the cornea. The human cornea consists of densely packed cells interspersed with pores of 2.0 ± 0.2 nm in diameter and consequently only drugs with a molecular weight of <500 da can penetrate (Hamalainen et al., 1997; Prausnitz and Noonan 1998). Lipophilicity also affects drug transfer, as drugs with high lipophilicity have high permeability but are cleared from the bloodstream quickly, whereas drugs with low lipophilicity have a low permeability but slow clearance from the bloodstream. This makes ocular drug delivery a complex clinical challenge.
Current methods assessing the penetration of ocular drugs rely on in vivo or ex vivo animal models (commonly rabbits or rodents), or in vitro cell culture. The use of in vivo rabbit models for ocular drug delivery is prevalent and highly contentious with over estimations of the human response due to species differences (Barar et al., 2009; Loch et al., 2012). This ex vivo model overcomes these limitations as the porcine cornea has similar permeability coefficients to the human eye (Loch et al., 2012), increasing the predictivity and relevance of the model as well as the reproducibility between assays.
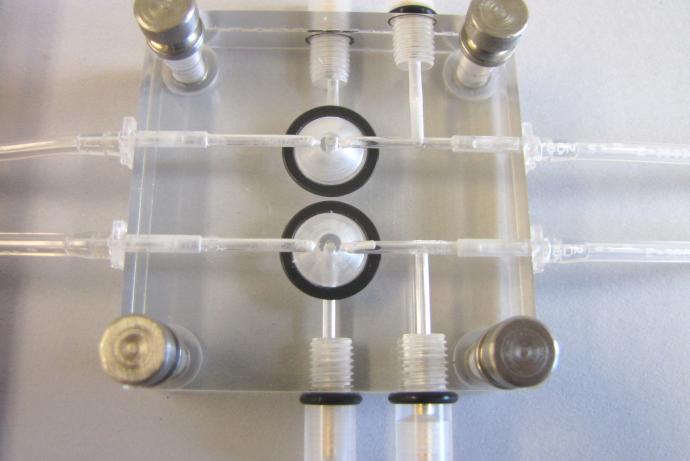
Current ex vivo models typically require expensive apparatus that can only hold a single piece of cornea (varying from 0.6 -15.1 mm in diameter) (Thiel et al., 2001; Toropainen et al., 2001), limiting the number of experiments it is possible to run simultaneously. This 96 well plate assay overcomes these limitations enabling multiple studies to be carried out concurrently using a reduced quantity of tissue (5 mm diameter). Even the small pieces of human corneal tissue available can be used, which, unlike current methods ensures no human tissue is wasted due to insufficient sample size.
In vitro models, using epithelial and endothelial cell lines from human/ rabbit to create a 3D culture, are time consuming to develop and epithelial resistance (a measure of barrier permeability) is not comparable to human corneal tissue (Barar et al., 2009; Shafaie et al., 2016). In vitro models also lack the necessary cell to cell interactions and changes in gene expression observed in vivo. Again, the ex vivo model proposed here overcomes these limitations with comparable epithelial resistance levels to human corneal tissue data found in literature (Loch et al., 2012; Shafaie et al., 2016).
References:
- Barar J, Asadi M, Mortazavi-Tabatabaei SA, et al. (2009) Ocular Drug Delivery; Impact of in vitro Cell Culture Models. J Ophthalmic Vis Res 4(4): 238-252.
- Hamalainen KS, Kananen K, et al. (1997) Characterization of Paracellular and Aqueous Penetration Routes in Cornea, Conjunctiva, and Sclera. IOVS 38(3): 627-634.
- Loch C, Zakelj S, Kristl A, et al. (2012). Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. European Journal of Pharmaceutical Sciences 47(1): 131–138. doi: 10.1016/j.ejps.2012.05.007.
- Shafaie S, Hutter V, Cook MT, et al. (2016). In Vitro Cell Models for Ophthalmic Drug Development Applications. BioResearch Open Access 5(1): 94-108. doi: 10.1089/biores.2016.0008.
- Thiel, M.A., Morlet N, Schulz D, et al. (2001). A simple corneal perfusion chamber for drug penetration and toxicity studies. Br J Ophthalmol 85(4): 450-3. doi: 10.1136/bjo.85.4.450.
- Prausnitz MR and Noonan J. (1998). Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 87(12): 1479-1488. doi: 10.1021/js9802594.
- Toropainen E, Ranta VP, Talvitie A, et al. (2001). Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci 42(12):2942-8.
To overcome the many barriers of the eye, topically applied drugs are given in large quantities to ensure a clinically relevant dose reaches the point of disease. Alternatively, injections can be used to bypass the barriers that are currently impenetrable by ocular drugs and treat diseases at the back of the eye such as age related macular degeneration. Finding methods to enhance penetration of ocular drug delivery are difficult and costly to assess.
Consequently, researchers at the University of Birmingham have developed a 96 well plate ex vivo porcine corneal assay to assess the rates of drug penetration and absorption through the cornea. This can be assessed by measuring the siRNA levels using qPCR, or by using HPLC or fluorescently tagged drugs. A range of controls have been developed within the assay to determine the integrity of the cornea with measured success of the penetration and adsorption of siRNA therapeutics. The assay can also use off cuts of human cornea, taking advantage of the valuable human tissue available to create a more predictive model. This fast, low cost method provides information for drug development and delivery, enabling the user to determine if the drug can pass through the cornea unaided or if it requires additional molecules to enhance penetration.
The ex vivo corneal assay could be used for multiple applications including, but not limited to, the following:
- Rapid and cost effective assessment of drug delivery through the cornea.
- Characterisation of delivery pathways of different classes of drugs through the cornea. This is achieved using fluorescently tagged drugs to determine which receptors/ junctions the drug binds to and consequently determine the delivery pathway.
- Development of a novel set of carrier molecules that can enhance the delivery of drugs across the cornea reducing the dose required and increasing the efficiency of treatments.
Industrial collaborators able to provide different classes of ocular drugs are sought to further assess how pore size and lipophilicity affect drug penetration through the cornea. The provision of drug penetration data held by industry would also be welcomed to enable further validation of the ex vivo corneal assay. Additionally, the researchers would greatly benefit from expertise and access to any drugs/ molecules capable of enhancing drug delivery through the cornea, providing the researchers with an insight into the drug transport mechanisms within the cornea.
In return, the researchers can assess the penetration of the collaborators ocular drugs through the ex vivo cornea model and help to validate a method of corneal drug delivery that could be applied by the collaborators.
Investigators commonly use in vivo rabbit models to assess corneal drug permeability, which often cause high levels of irritation and suffering and require ~100 animals per study. This Solution uses surplus porcine tissue from abattoirs to create an ex vivo corneal assay that is more comparable to the human eye in structure and permeability, increasing the applicability and predictive ability of the model (Loch et al., 2012). The 96 well plate format of the model enables four samples to be taken from one piece of tissue, reducing the number of eyes, and hence animals, required for each experiment by 75%. For example, the researchers lab alone uses ~100 rats per year to assess ocular drug delivery, but by using this ex vivo corneal assay these rats would be replaced entirely by tissue that is surplus to the food industry and readily available. If human corneal tissue can be obtained for the assay instead, then no animals would be required for these studies.
Using the ex vivo corneal assay as a primary screen in drug development enables identification of drugs with low corneal permeability, which can enable the researcher to remove the compound from development, reducing the number of compounds progressing to in vivo testing.
References:
- Loch C, Zakelj S, Kristl A, et al. (2012). Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. European Journal of Pharmaceutical Sciences 47(1): 131–138. doi: 10.1016/j.ejps.2012.05.007.