Newcells Biotech Ltd has developed an in vitro model of the retinal pigmented epithelium, to assess the safety and efficacy of novel ocular compounds. This technology could better predict the outcome in the human eye and reduce the number of animals used to assess the toxicity of ocular drugs. An end-user pharmaceutical company, able to provide access to ocular compounds and data with known in vivo/in vitro toxicity profiles, is sought to validate the model.
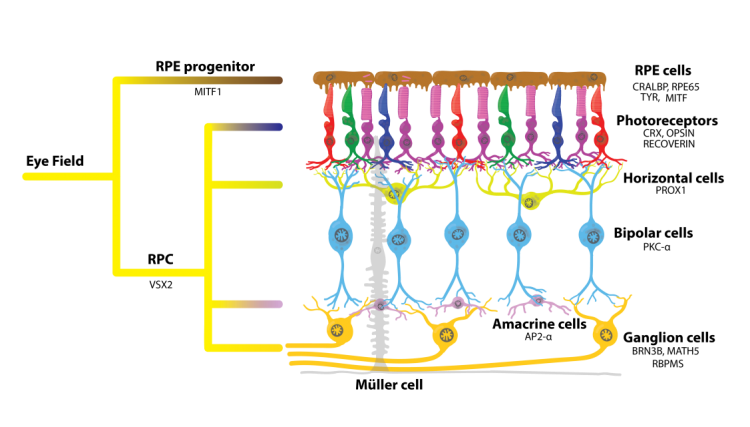
The retinal pigmented epithelium (RPE) is a single layer of pigmented cells that face the neurosensory retina (Figure 1). The RPE forms one of the blood-retina barriers of the eye and is vital for maintaining homeostasis of the retina (Straub, 2016). It is involved in light absorption, the secretion of growth factors, retinol cycling and production of pigment. One of the most important functions of RPE cells is phagocytosis of photoreceptor outer segments (POS). This process protects photoreceptors from oxidative damage and accumulation of lipofuscin, thereby maintaining a healthy sub-retinal environment. Disruption of one or more functions of the RPE can occur by mutation, environmental or pharmacologic interference, and lead to a range of disease states or toxic responses (Dellabella et al., 2015, Mecklenburg et al., 2007).
Animal models are widely used to assess the toxicity of topically and systemically applied drugs, even though the structure and physiology for commonly used regulatory species (rat and dog) is distinct from that seen in humans (Shafaie et al., 2016). There are protocols for culturing ex vivo human cells, but these cells are difficult to obtain and have inherent variability meaning studies can be difficult to compare (Kuznetsova et al., 2014). There is currently no established and validated in vitro model of human RPE, but the increased development of therapies targeting ocular diseases means there is an urgent need for this. The ability to study ocular drug toxicity and pharmacodynamic properties in a well characterised, high-throughput and human cell based assay would significantly improve industry’s ability to study drug safety and efficacy.
Figure 1. Schematic of the human retina showing position of the RPE layer
References
- Strauß O (2016). Pharmacology of the retinal pigment epithelium, the interface between retina and body system. Eur J Pharmacol 15: 787-84-93. doi: 10.1016/j.ejphar.2016.03.066.
- Dellabella A, Andres J (2015). Ophthalmic Toxicities of Systemic Drug Therapy. US Pharm. 40(6): HS19-HS24.
- Mecklenburg L, Schraermeyer U (2007). An Overview on the Toxic Morphological Changes in the Retinal Pigment Epithelium after Systemic Compound Administration. Toxicologic Pathology. 35(2): 252–267. doi: 10.1080/01926230601178199.
- Shafaie S, Hutter V, Cook MT, et al. (2016). In vitro Cell Models for Ophthalmic Drug Development Applications. BioResearch Open Access 5(1): 94-108. doi: 10.1089/biores.2016.0008.
- Kuznetsova AV, Kurinov AM, and Aleksandrova MA (2014). Cell Models to Study Regulation of Cell Transformation in Pathologies of Retinal Pigment Epithelium. Journal of Ophthalmology. 2014: 801787. doi: 10.1155/2014/801787.
Newcells Biotech Ltd has developed an in vitro model of the RPE derived from healthy and diseased human induced pluripotent stem cell (iPSC) lines, opening up the potential to assess safety in healthy cell models and efficacy in disease cells. The use of human iPSCs from single donors overcomes the limitations of primary cell culture, namely availability and variability.
The model is composed of a monolayer of RPE cells cultured in 24-well plate transwell inserts. This forms an epithelial barrier with Zona Occludens protein expression, which is required for maintenance of osmotic balance. Also, the trans-epithelial electrical resistance (TEER) is greater than 250 Ohms/Cm2, which is comparable to that seen in ex vivo human eyes (Quinn et al., 1992). The researchers have also demonstrated apical-basal polarity by evaluating the expression of some key proteins, including MERTK and Collagen IV (Figure 2) (Buskin et al., 2018). These characterisations confirm that the RPE monolayer is a close model of the in vivo human RPE.
Figure 2. Immunohistochemistry images of control and MertK- (F119) retinal pigmented epithelia derived from iPSC cell lines showing the polarised expression of key protiens including Collagen IV, DAPI and MertK.
Using flow cytometry, Newcells Biotech Ltd has established that the model is functional in terms of RPE cell phagocytosis of POS. It is possible to measure differences in phagocytosis function between RPE models derived from healthy and diseased patient iPSC lines (Buskin et al., 2018). This measure of phagocytic capacity can be used to monitor the effect of drugs and determine the ability of the RPE to support retinal function. Other readouts from the model include measurement of secreted protiens using an ELISA, monitoring TEER’s, and immunostaining. It is also possible to interrogate mechanisms of toxicity using single cell RNAseq to provide information on which pathways are induced by toxins. This model should be of great interest to the pharmaceutical industry, to assses both safety and efficacy of novel compounds.
References
- Buskin A, Zhu L, Chichagova V, et al. (2018). Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nature Communications 9(1):4234. doi: 10.1038/s41467-018-06448-y
- Quinn RH, Miller SS (1992). In Ion Transport Mechanisms in Native Human Retinal Pigment Epithelium. Ivestigative Ophthalmology & Visual Science. 33(13): 3513-27.
This model requires further validation against a range of xenobiotic substances to evaluate the capability of the RPE monolayer to assess drug safety to industrial standards.
An end-user pharmaceutical company, or consortium of companies, able to provide access to ocular compounds and data with known in vivo/in vitro toxicity profiles, is sought to validate the model. Cross-species data would be particularly valuable, as the researchers have access to non-human iPSC lines, including rat and non-human primate. In addition, input on assay endpoints of interest/value would ensure the model format is developed to best match the needs of the internal pre-clinical decision flow.
In return, the collaborators can access the technology on preferential commercial terms.
Information about IP
A limited freedom-to-operate search indicates that there are no obvious limitations to the commercialisation of the assay in its envisaged developed format. If IP is generated through this project, the intention is that this will be owned by Newcells, with any collaborator benefiting by negotiating preferential access terms. The ownership of the iPSC lines (healthy controls or diseased state) will remain with Newcells.
There is currently no validated in vitro model of the human RPE that can be used to assess drug safety or to model the various diseases that can effect the RPE. Assessment of overall ocular toxicity and disease modelling is typically carried out in retinal explant models from the mouse, rat or pig and a typical ocular safety assessment will use 20 animals per compound. Newcells’ assay can be used to carryout high volume screening of drugs, facilitating removal of unsuitable compounds prior to in vivo studies. Each compound removed from the pipeline will reduce the number of animals required for ocular toxicity studies by approximately 20.
Analysis of the current (Jan 2019) pipeline of drugs in discovery and preclinical stages that are targeting diseases of the eye revealed 438 programmes worldwide (Biopharma-Insight database, http://www.biopharminsight.com/). Newcells Biotech Ltd estimate that in the lead identification and optimisation stages of these projects, where 60-80% of all animals are typically used (Nuffield Council on Bioethics, 2005), approximately 50,000 animals would be required. This number is based on 2.5% of the compounds in the pipeline being assessed in vivo (requiring 20 animals per compound), of a pool of approximately 100,000 compounds distributed across early discovery to late pre-clinical. Recognising the timescale for the adoption of new testing regimes, Newcells Biotech Ltd predict that upon publication of positive validation data for their model, they could reduce animal usage in this field by approximately 10% (5,000 animals) in 2 years.
Reference
- Nuffieldbioethics.org. (2005). The ethics of research involving animals. Available at: http://nuffieldbioethics.org/wp-content/uploads/The-ethics-of-research-involving-animals-full-report.pdf (Accessed 1 May 2015).