OcuScience have developed new methods to preserve human retinas from organ donors and make them viable for research hours after death. The donor retina can be divided into multiple samples for intra-subject drug testing and screening. Combining OcuScience’s human tissue approach with their proprietary Handheld Multi-species ERG and Ex Vivo ERG Adapter, donor retina tissue which was once discarded can now be used in place of animals for a variety of eye research and drug development applications. OcuScience are looking to work with collaborators who have expertise in various diseases of the retina to provide direction and validation of the human disease models detailed in this Solution.
It is known from prior research (Frank and Dowling, 1968) that retinal tissue must be recovered and dissected soon after euthanasia to ensure a functional ERG response, a key source of quantifiable data to detect abnormal function of the retina. Therefore, there is a great need for and benefit of a human relevant retina model, which has been well described (NC3Rs CRACK IT Challenge: Retinal 3D, 2016; National Eye Institute 3-D Retina Organoid Challenge, 2017).
Laboratories around the world are investigating the use of stem cells and cell therapy to “build” a fully complex and functional human retina model. OcuScience has taken an alternative approach by developing tools to enable analysis of readily available and functional ex vivo tissue. The inherent advantages of using adult human donor tissue over the current state of research in stem cells and fetal tissue strategies provide a leap forward in comparison to existing models. For example, the dose response and therapeutic profile may be more predictive compared to animal in vivo and in vitro studies, since the model is utilising human tissue to investigate human disease. By accelerating the identification of target molecule physiological effect on the retina, retinal pigment epithelium (RPE), and similar neurological tissue, OcuScience will be able to efficiently identify candidate drugs for many human diseases.
References
- Frank RN, Dowling JE (1968). Rhodopsin photoproducts: effects on electroretinogram sensitivity in isolated perfused rat retina. Science 161(3840): 487-489. doi: 10.1126/science.161.3840.487.
- NC3Rs CRACK IT Challenge: Retinal 3D. http://nc3rs.org.uk/challenge-23-retinal-3d.
- National Eye Institute 3-D Retina Organoid Challenge. https://nei.nih.gov/3DROC.
Working with the Lions Eye Institute for Transplantation and Research in Florida, OcuScience have increased the timescale during which recovered tissue can be used and have determined the critical methods needed to capture retina function in ex vivo adult donor human eyes. OcuScience have an established methodology to obtain functional, reliable and repeatable ERGs. In particular, OcuScience have a means to sustain the retina in transport with a specialised device and nutrient solution which assists in preserving the function and tissue integrity; a unique dissection method that reliably yields a region-specific response; and a proven transretinal recording system that is unique in controlling multivariable influences on the data including electromagnetic noise, continuous nutrient perfusion, regulated physiological tissue support (temperature, perfusion rate and pH), and control of input electrode impedance. Each eye yields multiple samples which are processed into Human Retina Biosensors (HRB).
OcuScience has manufactured the versatile Handheld Multi-species ERG (HMsERGTM) for nearly a decade. To support the use of these HRB, OcuScience have now combined this with their Ex Vivo ERG Adapter to maintain viability while providing exposure of calibrated light to evoke composite responses from multiple cell types within the neural retina, e.g. from rod and cone photoreceptors to ganglion cells. Together with simultaneous data acquisition, OcuScience have a complete system to test nearly any species of neural retina.
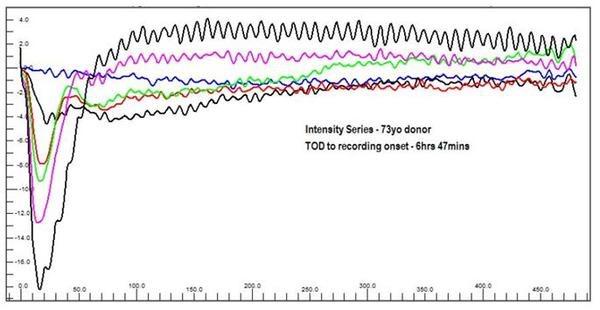
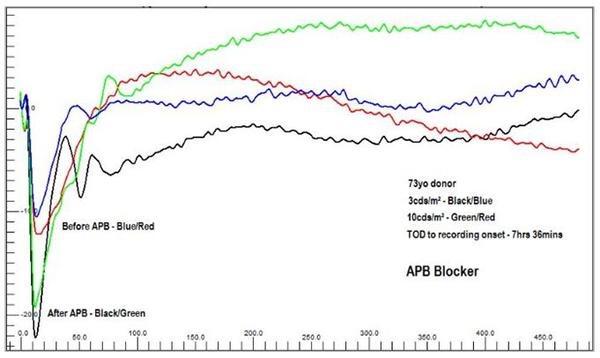
OcuScience has demonstrated proof of concept of testing pharmaceuticals for toxicology and pharmacological effects in ex vivo human tissue with several compounds known to affect ERG responses (Vinberg et al., 2014; Kolesnikov and Kefalov, 2012; Vinberg and Kefalov, 2015). The system stability and repeatability of the data make it possible to introduce compounds and quantitatively observe the change between baseline and treatment in a dose-dependent manner (see figures A and B below).
Figure A. Ex vivo Flash ERG intensity series of photostimulator evoked responses from a 73 year old male donor retina. This shows photoreceptor and retinal function are nearly identical to human in vivo retinal function morphology. Blue = 1 mcd.s/m-2, Black1 = 10 mcd.s/m-2, Red = 100 mcd.s/m-2, Green = 1,000mcd.s/m-2, Magenta = 3,000 mcd.s/m-2, Black2 = 10,000 mcd.s/m-2
Figure B. Example data showing change in response of HRB in the presence of 2-amino-4- phosphobutyric acid (APB), an on-bipolar pathway blocker, demonstrating data acquisition for drug testing. Baseline (Perfusion Only): Blue = 3,000 mcd.s/m-2, Red = 10,000 mcd.s/m-2. Treated (Perfusion with APB): Green = 3,000 mcd.s/m-2, Black = 10,000mcd.s/m-2.
A major advantage of this technology is the ability to test donor tissue from human diseases that are known to affect neurotransmission of photoreceptors, glial cells and ganglion cells such as Age-related Macular Degeneration (AMD), Diabetic Retinopathy and Retinitis Pigmentosa. This system has the potential to better predict safety and efficacy prior to human clinical trials as it uses ex vivo human retinal ERG responses, a closer analogy to a living human.
References
- Vinberg F, Kolesnikov AV, Kefalov VJ (2014). Ex vivo ERG analysis of photoreceptors using an in vivo ERG system.Vision Research 101:108-117. doi:10.1016/j.visres.2014.06.003.
- Kolesnikov AV, Kefalov VJ (2012). Transretinal ERG recordings from mouse retina: rod and cone photoresponses. Journal of Visualized Experiments 14(61) pii: 3424. doi:10.3791/3424.
- Vinberg F, Kefalov VJ (2015). Simultaneous ex vivo functional testing of two retinas by in vivo electroretinogram system. Journal of Visualized Experiments 6(99) e52855. doi:10.3791/52855.
Researchers:
OcuScience are looking to work with collaborators who have expertise in various diseases of the retina to provide direction and validation of the human disease models detailed in this Solution. OcuScience also seek alliances in using “big data” strategies to find candidate drugs and identify pharmacogenetic effects as a novel way to improve safety and efficacy.
OcuScience wants to share their knowledge about ex vivo ERG technology. They provide a variety of ways for researchers to learn how to utilise this technology specifically in the researchers area of expertise. OcuScience also provide the tools to researchers to conduct preliminary ex vivo studies in their own laboratories, prior to testing their compounds in the human retina biosensor.
Clients:
OcuScience is starting a contract research service focused on processing human donor retina for preclinical research and drug testing. OcuScience are fully prepared to test client compounds to their specification, conduct ERG functional testing, and structural analysis using OCT. OcuScience expect to process 5,000 HRB in the first year and plan to provide their service to any institution or pharmaceutical company to conduct exploratory experiments or test the functional effects of aa company’s compounds using OcuScience’s ex vivo HRB.
Donor Networks:
OcuScience would like to collaborate with experts in global organ donor networks. OcuScience intend to recover and process tissue worldwide to obtain pharmacogenomic data from various populations and ethnicities around the world, making future drugs safer and more effective.
Reduction:
This Solution promises to reduce the use of animals in the discovery of new ophthalmic drugs as well as screen out a significant number of compounds that would fail later in the pipeline, thus avoiding unnecessary animal testing. One estimation of the impact of other ex vivo ocular safety testing assays suggest a possible 10% reduction in live animal testing (Hood, 2008). Given the expanded ability of OcuScience’s HRB, they feel confident that more than 10% of animals used in retinal and CNS safety, toxicity, and dose-response analysis may be spared. This biosensor could also be used in basic research, reducing animal use and better informing the development of appropriate drugs for certain conditions.
Replacement:
The HRB could replace live animal testing where the effect of a compound is tested using electrophysiology. An estimated 3,000 articles are published annually that include data from animal testing of “back of the eye” and CNS effect of compounds. Assuming 50-100 animals could be replaced in each study using their technology, OcuScience project 150,000 to 300,000 may be saved annually by adoption of human donor retina testing.
Reference
- Hood E (2008). Alternative Test Models: Ocular Safety Assays Accepted. Environmental Health Perspectives 116(9): A381. doi:10.1289/ehp.116-a381.