This microfluidic platform enables tissue to be kept in a viable state maintaining architecture whilst the tissue continues to produce its normal products. Furthermore, it has been demonstrated that tumour tissues, taken from patient biopsies, respond to chemotherapy or external beam radiation in this device in a similar manner to a patients’ own in situ tumours when treated. Partners are sought to explore the application of these microfluidic devices in an industrial setting to assess efficacy and safety of drugs and other products, for testing novel drug delivery vehicles and establishing patient-specific drug approaches.
The literature is replete with studies using immortalised cell lines on-chip. These techniques have their uses, but they do not represent solid tumour structure (they are potentially of value for leukaemia studies however). A number of others have used spheroids as a 3D tumour model – some as co-cultures – and measured drug perfusion and metabolism etc. In respect of human or animal tissue on-chip the most studied area is in neuroscience, where brain slices (100-200 µm thick) have been used to study nerve biology.
Perhaps the nearest competitor to tumour biopsies on-chip for selecting the most efficacious treatment would be the patient-derived xenografts (PDX) in mice, also termed cancer avatars. In the latter case cancer biopsies are taken from the patient, established and then propagated in immunodeficient mice. Once sufficient tumour-bearing animals are established these are then treated with different therapeutic regimens with the aim of identifying the most efficacious on a personalised basis. The data from the small number of studies completed is highly encouraging in terms of predictive value and clinical response, however the main drawbacks are the time taken for tumours to be established, cost and welfare burden for the relatively high number of animals involved for each patient to be studied.
Whilst a vast amount of research can be done using single cell lines, co-cultures, spheroid and animal models (syngeneic and xenograft) none of these truly reflect the in vivo human environment. There are many benefits of using human tissue directly: the biopsies are purely of human origin, the analysis is rapid, no animals are used and the overall cost of fabricating and using these bespoke devices is low. Furthermore, the potential patient benefit would be in receiving the most effective treatment as a first line therapy which should lead to improved outcomes, with less ineffective treatment associated with side effects and the need for further clinical investigations. However, the major problem with primary culture of tissue biopsies is the need for effective perfusion with appropriately gassed media and continuous waste removal.
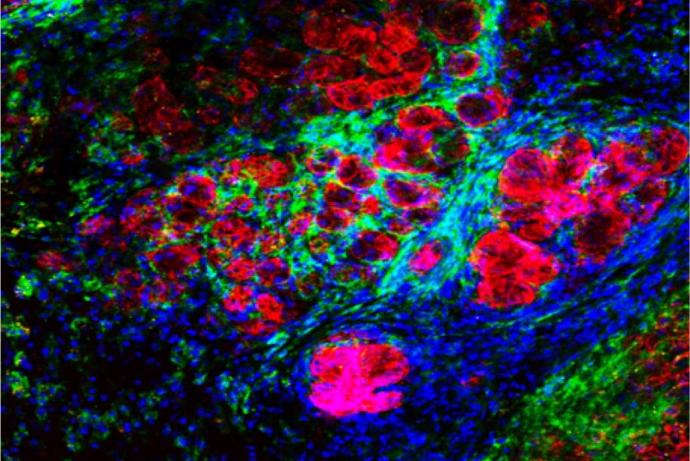
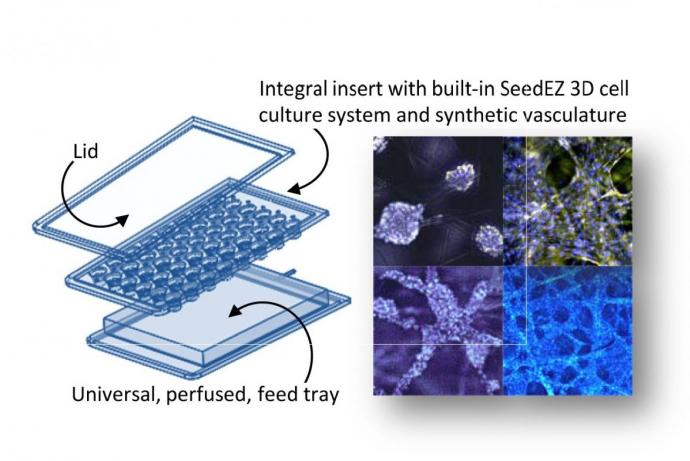
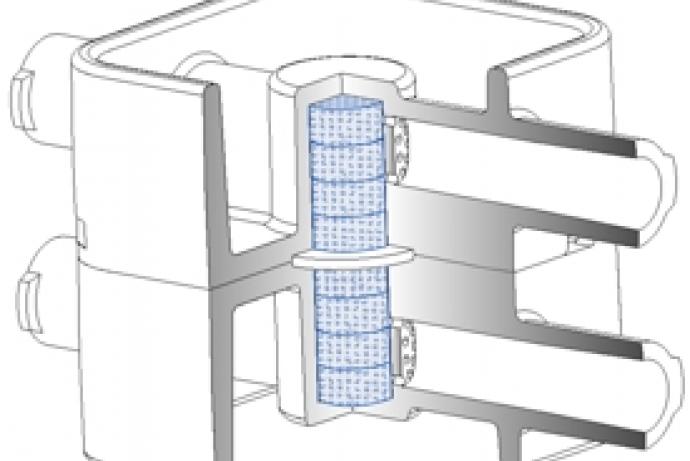
Researchers at the University of Hull have developed customised microfluidic systems to study tumour biology. Microfluidics is governed by laminar flow and diffusive mixing, identical characteristics that operate within capillaries controlling metabolite uptake, gaseous exchange and waste removal. This technology allows the researchers to study specific cellular and molecular interactions in real-time in a tissue biopsy, rather than using disaggregated cells. Multiple tissue types can be maintained across several chips and the channels designed to allow media and metabolites to pass from one tissue to the other. This capability is of particular value for analysis of liver metabolism and other off-target tissue effects.
A key consideration is the size and shape of the tissue biopsy, as the surface area to volume ratio needs to remain high enough to allow efficient transfer of materials and prevent hypoxia or a build-up of toxic metabolites. However, the biopsy needs to be large enough to represent the true functions of the tissue from which it was taken (Carr et al., 2014; Cheah et al., 2010; Hattersley et al., 2012; Hattersley et al., 2008; Hattersley et al., 2011; Hattersley et al., 2013; Shaw et al., 2010; Sylvester et al., 2013; Webster et al., 2010; Woods et al., 2011).
Personalised treatment for solid tumours would be one area that the researchers wish to pursue. Patient samples would be taken at resection and subjected to multiple chemo- and chemoradiotherapy treatments on chip. Tissue would then be subject to a series of proteomic and genomic analyses to understand the tissue response to different drug levels and times of exposure. The microfluidic platform would also allow studies of the off-tissue effects of drugs using non-tumour tissue in separate and/or integrated microfluidic devices in parallel with the malignant target.
A second application of the technology would be to use the dual-flow chip that allows different faces of tissue, e.g. epithelial versus stromal layers, to be independently perfused and treated. This chip is currently being used to study inflammatory bowel disease.
To reduce rather than immediately replace animal use, it is possible to study xenograft slices from existing models in the microfluidic devices. Currently each PDX animal is used individually for testing a specific drug treatment. Using precision cut slices it would be possible to generate five to 15 slices per animal for drug testing in a microfluidic device, lowering the number of animals required.
References
- Carr SD, Green VL, Stafford ND, et al. (2014). Analysis of Radiation-Induced Cell Death in Head and Neck Squamous Cell Carcinoma and Rat Liver Maintained in Microfluidic Devices. Otolaryngol Head Neck Surg 150(1): 73-80. doi:10.1177/0194599813507427.
- Cheah LT, Dou YH, Seymour AM, et al. (2010). Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab on a Chip 10(20): 2720-6. doi:10.1039/c004910g.
- Hattersley SM, Sylvester DC, Dyer CE, et al. (2012). A Microfluidic System for Testing the Responses of Head and Neck Squamous Cell Carcinoma Tissue Biopsies to Treatment with Chemotherapy Drugs. Ann Biomed Eng 40(6): 1277-88. doi:10.1007/s10439-011-0428-9.
- Hattersley SM, Dyer CE, Greenman J, et al. (2008). Development of a microfluidic device for the maintenance and interrogation of viable tissue biopsies. Lab on a Chip 8(11): 1842-6. doi:10.1039/b809345h.
- Hattersley SM, Greenman J, Haswell SJ (2011). Study of ethanol induced toxicity in liver explants using microfluidic devices. Biomed Microdevices 13(6): 1005-14. doi:10.1007/s10544-011-9570-2.
- Hattersley SM, Greenman J, Haswell SJ (2013). The application of microfluidic devices for viral diagnosis in developing countries. In: Methods in molecular biology (Ed. Jenkins G, mansfield CD). Humana Press, Totowa, NJ. 949: 285-303. doi:10.1007/978-1-62703-134-9_19.
- Shaw KJ, Docker PT, Yelland JV, et al. (2010). Rapid PCR amplification using a microfluidic device with integrated microwave heating and air impingement cooling. Lab on a Chip 10(13): 1725-8. doi:10.1039/c000357n.
- Sylvester D, Hattersley SM, Stafford ND, et al. (2013). Development of microfluidic-based analytical methodology for studying the effects of chemotherapy agents on cancer tissue. Curr Anal. Chem. 9(1): 2-8.
- Webster A, Dyer CE, Haswell SJ, et al. (2010). A microfluidic device for tissue biopsy culture and interrogation. Analytical Methods 2(8): 1005-7. doi:10.1039/C0AY00293C.
- Woods J, Docker PT, Dyer CE, et al. (2011). On-chip integrated labelling, transport and detection of tumour cells. Electrophoresis 32(22): 3188-95. doi:10.1002/elps.201100172.
The team at the University of Hull has a unique combination of biological, engineering and clinical expertise, as well as facilities for collecting and exploiting biological samples on bespoke series of microfluidic devices. The researchers at the University of Hull have excellent links with clinicians across the UK and can access appropriate samples if necessary and could install analysis devices at additional test sites.
Collaborations are sought with pharmaceutical, consumer product/cosmetic, and/or chemical companies to:
- Allow pseudo-clinical trials to be undertaken on new, or novel combinations of existing drugs, for various tumour types to establish efficacy on a personalised basis.
- Establish robust devices for maximising data output from human xenograft (PDX) tumour slices.
- To explore the utility of these devices for undertaking toxicology studies across a range of industries.
Finally, the researchers would be keen to work with clinical device manufacturers who wish to integrate analysis methodologies on-chip to provide novel devices capable of near-patient testing. The researchers have extensive expertise in the use of luminometry, spectrometry, PCR analysis and flow cytometry on-chip through links with the Department of Chemistry.
Information about IP
The IP landscape for microfluidic devices is extremely dense and the use of tissue in the ways exploited at Hull are considered non-patentable in themselves. However the development of novel devices, integrating specific analysis methodologies would be potentially patentable. The University does hold a number of patents on certain device designs.
Generating PDX is an inefficient process, requiring relatively large numbers of animals to be grafted to ensure sufficient numbers are established for each individual. For example, biopsies from 29 sarcoma patients were used to create PDX, but only 16 (55%) generated PDX models able to provide data of any clinical value (Stebbing et al., 2014). Combining novel chip-based devices with ex vivo tumour tissue slices, could markedly reduce this animal use as it is possible to obtain multiple slices from each tumour bearing animal that can each be used for conducting multiple analyses and drug testing.
More generally, most current clinical research uses animal disease models that are treated with test substances. Despite many known limitations these animal models remain commonly used, and for drug testing are often explicitly required. In 2013 the number of animals used in cancer research was 500,769, a 13% increase from the previous year (Statistics of scientific procedures on living animals, 2013). The adoption of microfluidic technology to maintain patient biopsies could offer a viable replacement option for many animal models used in basic research and drug efficacy and safety testing; potentially reducing the number of compounds entering regulatory required in vivo testing.
References
- Statistics of scientific procedures on living animals, Great Britain 2013.
- Stebbing J, Paz K, Schwartz GK, et al. (2014). Patient-derived xenografts for individualized care in advanced sarcoma. Cancer 120(13): 2006-15. doi:10.1002/cncr.28696.