Osteo-chip
The aim of this Challenge was to develop an in vitro that could recapitulate the human osteoarthritic join with multiple cell types for research and drug development in osteoarthritis. The model needed to provide mechanistic insight into disease development, including pain pain development through biomarker detection.
To address the requirements of the Challenge, a team led by Professor Anne Dickinson, Alcyomics Ltd, developed an osteoarthritis 3D model of cartilage incorporating immortalised osteoblasts and chondrocytes. Introducing injury to this in vitro model releases biomarkers and molecules characteristic of the cartilage damage seen in osteoarthritis, enabling the efficacy testing of novel therapeutics.
CRACK IT Challenge webinar: An in vitro model of the human osteoarthritic joint
In March 2023, we hosted a webinar to provide an overview of the 3D in vitro model of Osteoarthritis that the team at Alcyomics developed through the Osteo-chip Challenge. Speakers included Anne Dickinson (Alcyomics), Kenny Dalgarno (Newcastle University) and Mahid Ahmed (Alcyomics). The recording is now available to watch below.
Challenge Completed
Through the Osteo-chip Challenge, Professor Anne Dickinson from Alcyomics Ltd have developed an in vitro 3D model of cartilage, that will allow end-users to mimic cartilage damage seen in osteoarthritis.
Publication
Zhang H, Whalley RD, Ferreira AM and Dalgarno K (2020). High throughput physiological micro-models for in vitro pre-clinical drug testing: a review of engineering systems approaches. Progress in Biomedical Engineering 2 022001. doi.org/10.1088/2516-1091/ab7cc4.
Technology update
Alcyomics acquire the cell printer to create their 3D cell models.
Phase 2 awarded
A team led by Professor Anne Dickinson from Alcyomics Ltd has been awarded £999,996 to deliver the project: Microfluidic Automated Platform for Osteoarthritis Drug Screening (MA-PODS).
Phase 1 awarded
Three Phase 1 Awards were made to project teams led by:
- Dr Astrid Bakker, Stichting VU, Vrije Universiteit Amsterdam - ACTA, £99,973.
- Professor Kenneth Dalgarno, Newcastle University, £96,672.
- Dr Deborah Mason, Cardiff University, £99,820.
Challenge launched
Sponsored by GSK and co-funded by Versus Arthrtis and EPSRC, the Osteo-chip CRACK IT Challenge aims to create an in vitro model to recapitulate the human osteoarthritic joint.
Background
Osteoarthritis (OA) is the most common musculoskeletal disease affecting nine million individuals in the UK alone. A chronic disease, OA develops with a pathology characterised by cartilage loss, synovial inflammation, subchondral bone sclerosis and cyst formation, and osteophytosis. This pathology causes pain and a loss of a range of movements and ultimately results in joint failure (Dieppe et al., 2005).
Factors contributing to the development of OA include inflammation, trauma, ageing, obesity and genetic disposition (Heidari et al., 2011). Treatment for OA targets three main areas: pain relief, restoration of function and prevention of disease progression. The development of new therapies not only requires an understanding of the pathophysiology of OA, but also the associated biomechanical, inflammatory, genetic and environmental risk factors (Hunter, 2011).
In the investigation of treatments for OA, a range of humanised mouse, rat and guinea pig models have been developed to study different combinations of output measures such as pain, synovitis and cartilage degeneration, but these currently are not standardised making it difficult to compare results between studies. There is inter-laboratory variability even when the same models are used, making reproducibility of findings difficult (Vincent et al., 2012). Differences in animal and human physiology, particularly with regard to the immune system, can impact on the translation of preclinical findings to the clinic. In addition, the load bearing mechanics of joints in animal models which are quadrupeds presents further potential hurdles to translation.
Animal models of OA make it possible to study the whole joint structure with the majority of models focusing on the knee joint. These fall into two main categories: those that model painful behaviour (typically intra-articular monosodiumiodoacetate injection (MIA) and surgical models) and those that model disease progression through cartilage degradation (spontaneous and surgical models). Models recapitulating other aspects of the disease are less well developed – for example, there is a scarcity of papers published on bone changes in OA models and changes in other tissues including, the synovium, capsule, joint adipose tissue (infrapatellar fat pad), muscles, ligaments and tendons.
A typical surgical model of joint disruption is carried out for 12 weeks following surgery (with mice operated on at ten weeks of age) and thus a minimum of six months per experiment is required (Miller et al., 2012). Joint damage over the duration of the experiment can be extensive, leading to ambulatory deficiencies in the animals and in addition, the rodents experience high levels of chronic pain associated with the disease.
The welfare concerns, long duration and significant costs of running these whole animal models, along with their limited translation to the human disease, means that academic and industry researchers are seeking higher throughput in vitro models for both chemical and surgical induction (as assessed by histology or biomarkers).
This CRACK IT Challenge aims to develop an advanced in vitro model of the human osteoarthritic joint that will:
- Reduce the number of animals used in preclinical OA drug development and academic research by providing an alternative to the animal models.
- Improve the predictivity of preclinical modelling to humans through more extensive use of human tissues and/or cells.
- Provide a robust and reliable tool for development of potential disease modifying OA drugs.
3Rs benefits
Arthritis is an active area of preclinical study with a range of animal models used in academia and industry. No model replicates the human condition precisely, either in terms of pathogenic mechanism or response to treatment, and there is no consensus as to which model best recapitulates the human disease. Often researchers will run multiple models at the same time to assess reproducibility, therefore increasing the number of animals used (Ashraf et al., 2014). Moreover, if an OA model is being used to study pain or test analgesics, the experiment may require researchers to not administer post-operative analgesics, resulting in pain which may last for up to ten days (Kelly et al., 2013).
Chemical and surgical induction of OA in animals (MIA, meniscal transection (MNX), disruption of the medial meniscus (DMM) and anterior cruciate ligament transection (ACLT)) causes sustained and chronic pain, which progressively worsens with study length, typically 14-28 days for MIA injection in rats (Kelly et al., 2013), but up to 16 weeks for DMM surgery in mice (Miller et al., 2012). This ongoing pain and concomitant joint destruction significantly impairs animal movement and can have negative effects on other normal behaviours, such as feeding and nest building.
Although spontaneous models of OA do not involve any post-operative pain, there is significant variability in the development and severity of disease and models have to be used for significantly longer periods for example up to three years for Dunkin Hartley guinea pigs (McDougall et al., 2009) and 12 months for STR/ort mice (Kyostio-Moore et al., 2011).
Studies for preclinical arthritis models often require up to five groups per study with at least two treatment arms, using up to 40 animals per study. For a pharmaceutical company carrying out these types of studies, both in-house and outsourced, the total numbers of animals used per year can be in excess of 700 animals.
If solved, this Challenge will significantly reduce the number of animal models used in the study of OA and provide more human-relevant information such as novel biomarkers that may be used to refine any animal studies that are still needed through using earlier and more humane endpoints.
Phase 1 winners
- Dr Astrid Bakker, Stichting VU, Vrije Universiteit Amsterdam - ACTA, £99,973.
- Professor Kenneth Dalgarno, Newcastle University, £96,672.
- Dr Deborah Mason, Cardiff University, £99,820.
Phase 2 winner
Project team lead by:
- Professor Anne Dickinson, Alcyomics Ltd £999,996.
Full Challenge information
Assesment information
Animal models used in the study of osteoarthritis (OA) can cause significant suffering. The data collected using in vivo models generates significant variability and are often not predictive of the disease in humans.
The team at Alcyomics, led by Professor Anne Dickinson, co-funded by Versus Arthritis and EPSRC with support from Challenge Sponsor GSK, have developed an osteoarthritis 3D model of cartilage incorporating immortalised osteoblasts and chondrocytes, which is designed to overcome the limitations of in vivo models. Introducing injury to this in vitro model releases biomarkers and molecules characteristic of the cartilage damage seen in osteoarthritis, enabling the efficacy testing of novel therapeutics.
The standardised, reproducible 3D model developed through this Challenge provides human-relevant data and can be used to replace the use of small animals as models in the study of OA disease progression and drug development.
This product has been developed and led by the team at headed by Emeritus Professor Anne Dickinson, CEO of Alcyomics, in collaboration with Professor Paul Genever, University of York, Dr Georg Felchinger, University of Leeds, Professor Kenny Dalgarno, Newcastle University and Dr Richard Pearson, University of Nottingham.
For more information about Alcyomics please visit their website.
A 3D model of the human osetoarthritic joint
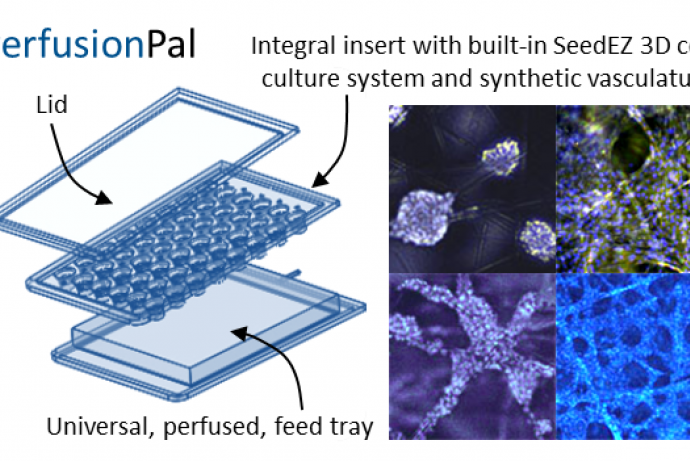
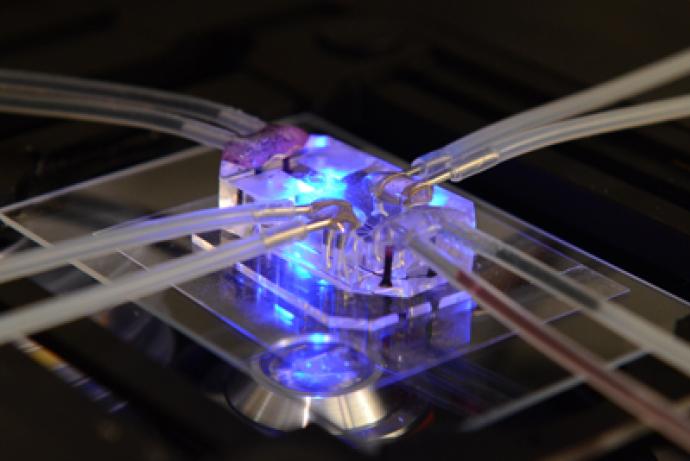
Alcyomics Ltd have developed an osteoarthritis (OA) 3D cartilage model incorporating immortalised osteoblasts and chrondrocytes. Designed to recapitulate the human joint, the model is constructed with a 3D scaffold to create a defined bi-layer containing both cartilage and bone cell lineages.
Mesenchymal stem cells (MSC) are seeded and differentiated in the 3D environment to form mature chondrocytes. Osteoblasts are bioprinted onto the mature chondrocyte construct, which allows for reproducible, high-throughput manufacturing. When damaged, the model releases biomarkers or molecules characteristic of cartilage and/or bone damage to recapitulate OA in vitro. It is based in a 96 well scaffold format and is suitable for drug screening, for example to test the efficacy of new drugs and formulations to limit OA disease phenotypes.
At present, cartilage from animals is primarily used in OA research due to the lack of in vitro alternatives for drug testing. This model has the potential to directly replace animals in drug discovery, giving reproducible results that are more human-relevant and increasing the efficiency of the identification of new drug targets and molecular pathways associated with cartilage repair.
Features



Access the technology
For more information on the model and how to access the technology, please see the Alcyomics website or contact Alcyomics directly.