FibromEd Ltd have developed HepatoinformTM, an iPSC derived platform for assessing hepatotoxicity. They are keen to develop partnerships with the pharmaceutical industry, contract research organisations and the chemical industry to further validate the assay. The company is open to exploring a variety of options for partnerships from companies interested in screening their internal compounds through to companies who provide sophisticated screening platforms for drug discovery, chemical standards and research materials for others.
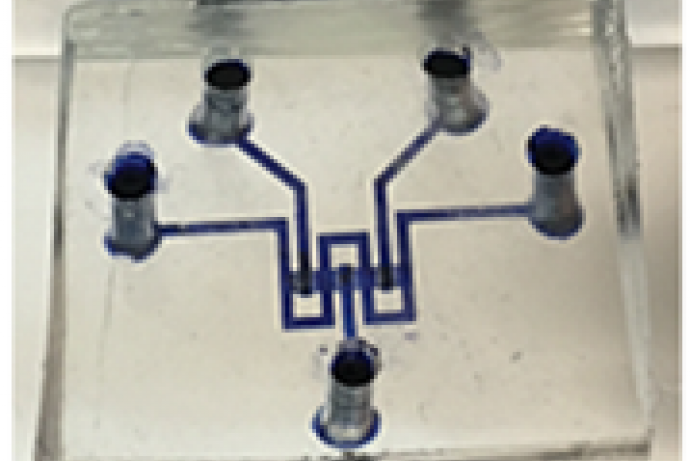
Through CRACK IT Solutions FibromEd partnered with Kirkstall Ltd and were awarded Solutions funding to optimise the culture of stem cell-derived hepatocytes in fluid shear stress chambers and assess the cells response to a known hepatotoxin.
The pharmaceutical industry uses a number of different in vivo and in vitro approaches to assess the potential for new drugs to cause liver injury (hepatotoxicity). However, with hepatotoxicity being the second most common cause of drug failure, there are clear limitations in the ability of these models to predict human toxicity. The development of more predictive human safety models incorporating the influence of drug metabolism is urgently required to facilitate development of safer and more cost-effective medicines.
FibromEd Ltd have developed a novel stem-cell derived hepatocyte system, Hepatoinform to predict human drug toxicity and drug induced liver injury. Hepatoinform is derived from a renewable source (pluripotent stem cells) of known genotype and therefore addresses the current worldwide shortage of human hepatocytes. This human-based system is predictive of human hepatocyte toxicity notably identifying toxicities with IC50 values comparable to freshly isolated human hepatocytes (manuscript in preparation). FibromEd Ltd therefore believe their model has significant potential in reducing the use of animals in safety testing and drug development, the current levels of human drug attrition and the costs of human drug development. Hepatoinform applications range from toxicity screening tools, to the prediction of idiopathic drug-induced liver injury, providing chemical standards for industry and other genotype-specific effects.
FibromEd Ltd have been working with the pharmaceutical industry to validate the added value of their cell-based system using a panel of compounds that displayed human hepatotoxicity and operate through known cellular mechanisms. These studies have proved very successful in predicting which compounds would be toxic in humans and also offer the possibility to generate novel chemical standards. FibromEd Ltd are therefore keen to extend the depth of understanding, predictive nature and utility of Hepatoinform using an extended range of compounds. In addition to FibromEd Ltd’s current capabilities, they can also custom build human models to suit the client’s requirements.
In the development of new medicines, toxic effects in the liver are always an important consideration. The current tests are conducted in animal models. Statistics published by the Home Office (2010) showed that in the UK, just over 210,000 animals were used in toxicology research and safety testing. In the short term the use of Hepatoinform has the potential to reduce animal use by preventing compounds that are destined to fail in the clinic due to liver toxicity undergoing tests in animals. In the long term and with further validation this approach has the potential to replace animal use in determining liver toxicity in some instances.
Overview | Impact | Publication
Overview
The team is keen to evolve the platform to culture the cells in an environment that better mimics liver physiology in vivo. This includes the provision of a stable environment with the constant delivery of nutrients, removal of waste products and presentation of key liver factors using fluid flow across their cells. Evidence exists supporting the importance of fluid shear stress in other cell types, but it has been lacking in the case of hepatocytes.
Through CRACK IT Solutions, FibroMed identified a partner, Kirkstall Ltd, able to provide culture chambers that permit exposure to fluid shear stress, in two and three dimensions. Supported by CRACK IT Solutions funding, both teams embarked on a project to:
- Optimise the seeding of stem cell-derived hepatocytes in fluid shear stress chambers.
- Optimise the level of fluid shear stress that stem cell-derived hepatocytes are exposed to.
- Assess the response of the stem cell-derived hepatocytes to a known hepatotoxin following exposure to fluid shear stress.
Impact
To assess the effect of flow on metabolic activity, 2D- and 3D-cultured stem cell-derived hepatocytes were transferred into serially-connected chambers of the Kirkstall Ltd Quasi-Vivo® system and exposed to a range of fluid shear stress of 2.9 to 6.5×10-5 dynes/cm2 using a peristaltic pump. Metabolic cytochrome P450 (CYP) activity of two isoforms of Cyp1A2 and Cyp2D6 were measured to evaluate the effect of flow on stem cell-derived hepatocyte metabolic activity, and the response to a known hepatotoxin supplied by the pharmaceutical company Bristol-Myers Squibb.
These studies highlighted that stem cell-derived hepatocytes are able to tolerate fluid shear stress and this resulted in improved Cyp2D6 metabolic activity. Cyp1A2 metabolic activity was differentially regulated under these conditions and this will be the subject of future investigation. Importantly, prototype model sensitivity is improved in response to flow, resulting in improved pharmaceutical grade compound metabolism.
The teams have successfully developed a scalable and predictive 2D and 3D human hepatocyte prototype model to study the influence of fluid shear stress on stem cell-derived hepatocyte function. The knowledge obtained from this project provides proof-of-concept that fluid shear stress improves hepatocyte phenotype and sensitivity to a known drug. Therefore the cell-based models generated in this programme of work will be more sensitive tools for use in human drug safety trials and may obviate the need for animal use in safety testing experiments.
The full output of this study has recently been published in Archives of Toxicology.
Publication
Rashisi H, et al. (2016). Fluid shear stress modulation of hepatocyte-like cell function. Arch Toxicol 90(7): 1757-61 doi:10.1007/s00204-016-1689-8.