DRGNET
The aims of this Challenge were i) to put in place a system whereby high quality and viable dorsal root ganglion neurones could be supplied to both industrial and academic researchers and ii) to increase understanding of the human system and facilitate drug target identification and pharmacological testing of novel pain therapeutics.
To solve this Challenge, the team lead by Professor Andrew Hart at NHS Greater Glasgow & Clyde, in collaboration with Grunenthal and Metrion Biosciences Ltd, has developed an approach for the ethical collection of human DRG material. They have demonstrated that it is possible repeatedly isolate, culture, and transport viable, pain relevant human neurons to distant laboratories whilst still working within the legal and regulatory framework for research using human tissue. They have also demonstrated that it is possible to generate robust and reproducible functional and pharmacological responses in these cells across the partner sites.
Conference presentation
Harvard Medical School Annual Bioethics Conference 2018 (Boston, USA)
Brain stem death: From heart transplants to therapeutic pain research – expanding the role of donated tissue.
Society for Neuroscience 2017 Annual Meeting (Washington, USA)
Development, validation and functional characterisation of primary cultures of sensory neurons derived from adult human dorsal root ganglia.
Challenge completed
Professor Andrew Hart in collaboration with Grunenthal and Metrion Biosciences Ltd has developed an approach for the ethical collection of human DRG material. They have demonstrated that it is possible repeatedly isolate, culture, and transport viable, pain relevant human neurons to distant laboratories whilst still working within the legal and regulatory framework for research using human tissue. They have also demonstrated that it is possible to generate robust and reproducible functional and pharmacological responses in these cells across the partner sites.
The team will continue to work together to further characterise the cells and expand the number of collection sites to increase the amount of human DRG material available to the research community.
Publication
Skotis GD, Cumming DRS, Roberts JN, et al. (2015) Dynamic acoustic field activated cell separation (DAFACS). Lab on a Chip 15(3): 802-810. doi:10.1039/C4LC01153H.
First human DRGs collected and shipped to project partners
Phase 2 awarded
A team led by Professor Andrew Hart, NHS Greater Glasgow & Clyde has been awarded £749,976 to deliver the project: Developing an ethical, sustainable, human sensory neuron culture model for use in therapeutic pain research.
Phase 1 awarded
Two Phase 1 Awards were made to project teams led by:
- Professor Praveen Anand, Imperial College London, £99,359.
- Professor Andrew Hart, NHS Greater Glasgow & Clyde, £99,997.
Challenge launched
Sponsored by Grünenthal and Pfizer, the DRGNET Challenge aims to put in place a system whereby high quality and viable dorsal root ganglion neurones can be supplied to both industrial and academic researchers to increase understanding of the human system and facilitate drug target identification and pharmacological testing of novel pain therapeutics.
Background
Chronic pain is a common problem affecting approximately one in five adults across Europe. It has a substantial impact on patients’ quality of life and is associated with physical and social disability, as well as psychological distress. Although a variety of analgesic agents are available many patients remain refractory to these treatments because of inadequate pain relief or intolerable side effects. There remains the need for the development of additional treatments with better efficacy and/or toleration profiles.
Pain signalling is transmitted by sensory neurones, a specialised neural population conveying sensory information from the periphery to the central nervous system. The cell bodies of somatic sensory neurones lie out with the spinal column in a series of ganglia, termed dorsal root ganglia (DRG). Key ion channels and receptors expressed in the nociceptive sensory neural population are targets for the development of novel pain therapeutics. The pain field has seen a number of high profile failures through lack of efficacy at Phase II in recent years, and development of more predictive in vitro models is key to addressing this.
The pain field suffers from a lack of access to viable human DRG material. Post mortem material is rarely viable enough to allow physiological profiling and no robust immortalised DRG lines have been developed. A few publications have demonstrated the utility of freshly isolated human DRG material for small scale electrophysiological studies. Much of the in vitro work is carried out on DRG isolated from preclinical species (rodent, dog and non-human primate (NHP)), and the absence of predictive in vitro assays results in increased in vivo exploratory experimentation in preclinical species. Recent work has aimed to derive human nociceptive neurones from pluripotent stem cells, opening up the possibility of generating induced pluripotent stem (iPS) cells from patients with chronic pain; this work suffers from a lack of comparator studies with human DRG neurones.
A reliable source of human DRG material would allow more physiologically relevant information to be generated in the human sensory neural system for drug target identification and drug discovery, and the development of more refined stem cell-derived strategies for a higher throughput approach.
3Rs benefits
Access to a reliable source of primary human DRG neurones will lead to several 3Rs benefits:
- Replacement of the use of animal DRGs (rat, mouse, dog, NHP) with human DRGs in basic research and drug development.
- More physiologically relevant in vitro models will lead to a reduction in the use of in vivo models e.g. scale back of in vivo exploratory programmes for target identification and selection triage.
- Better understanding of human DRGs will facilitate the development of iPS cell derived sensory neurons to use in high-throughput screening for candidate selection. Reducing the use of animals on compounds destined to fail later in development.
Phase 1 winners
Project teams led by:
- Professor Praveen Anand, Imperial College London, £99,359.
- Professor Andrew Hart, NHS Greater Glasgow & Clyde, £99,997.
Phase 2 winner
Project team led by:
Full Challenge information
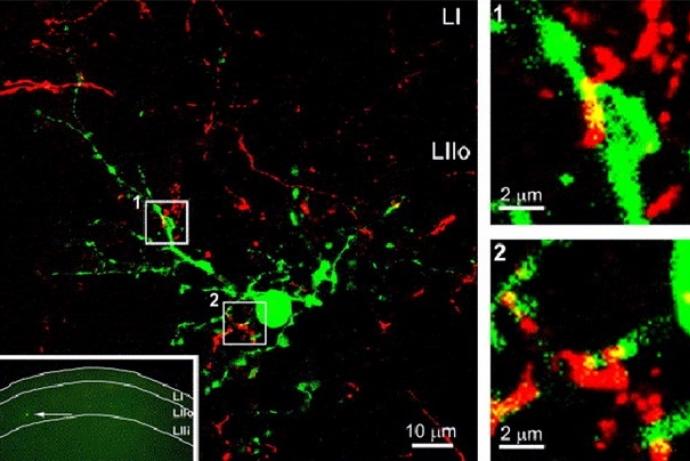
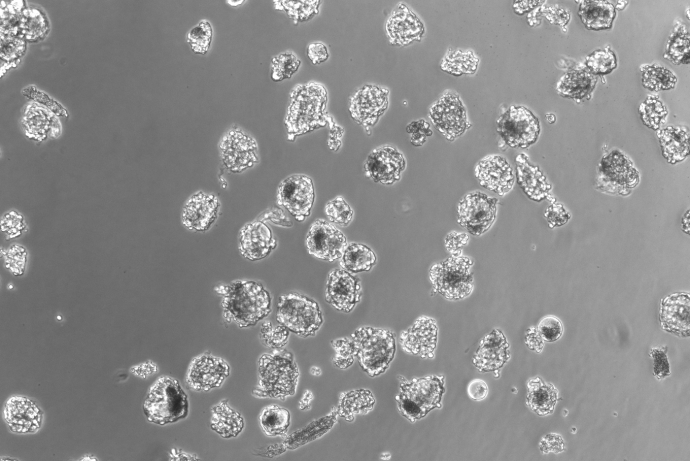
The DRGNet team, led by Professor Andrew Hart, has established an approach for the ethical collection and distribution of human dorsal root ganglion (DRG) neurons to facilitate drug target identification and pharmacological testing of novel pain therapeutics without using animals. This includes establishing an operational structure for harvesting human DRG material, and tissue retrieval and extraction processes which maintain tissue viability. Harvested cells are morphologically and phenotypically neuronal and the team has developed laboratory processes and culture protocols which maintain the stability of the neuronal phenotype for more than 40 days in culture.
The human DRGs have been transported to project partners in Scotland, Cambridge and Germany and maintain their viability and neuronal phenotype. They have been extensively characterised in terms of their electrophysiology, functionality and pharmacology, and respond reproducibly across sites, highlighting the potential for these human tissues to be integrated into research pipelines.
Professor Hart continues to work with the Challenge Sponsors to further characterise the human DRGs. He is also exploring opportunities for scaling up the volume of human tissue available to sustain research programmes and make DRG material more widely accessible.