IVIVE
The aim of this Challenge was to develop a model to interpret toxicity concentration response data generated in vitro in the context of relevant human exposures. The focus was to support systemic toxicity assessment as opposed to localised endpoints such as skin or eye irritation. This Challenge was set to deliver a new understanding of in vitro exposure parameters and how these relate to safe human doses.
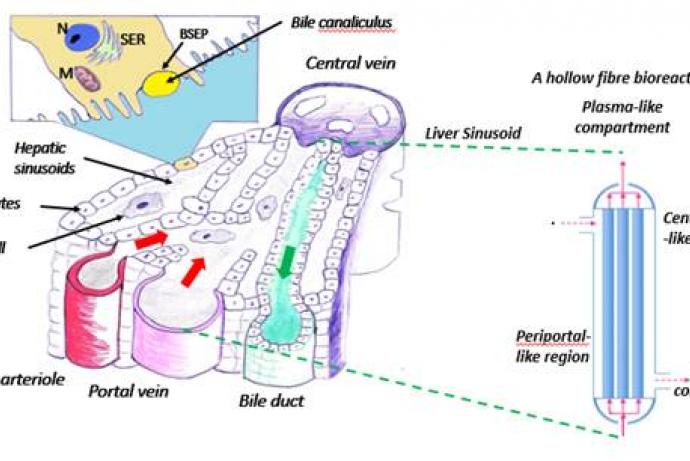
The multi-disciplinary team led by Dr Steven Webb at Liverpool John Moores University has developed a zonated hollow fibre bioreactor that more closely replicates the architecture and physiology of the liver for toxicology testing.
Publication
Tomlinson, L et al. (2019). In vitro Liver Zonation of Primary Rat Hepatocytes. Frontiers in Bioengineering and Biotechnology. doi.org/10.3389/fbioe.2019.00017.
Publication
Luetchford KA, Wung N, Argyle IS, et al. (2018). Next generation in vitro liver model design: Combining a permeable polystyrene membrane with a transdifferentiated cell line. Journal of membrane science 565: 425-438. doi.org/10.1016/j.memsci.2018.07.063.
NC3Rs workshop

Dr Steven Webb presented the outcomes of the IVIVE Challenge at the NC3Rs workshop: Applying exposure science to increase the utility of non-animal data in efficacy and safety testing.
Publication
Storm MP, Sorrell I, Shipley R, et al. (2016). Hollow fiber bioreactors for in vivo-like mammalian tissue culture. Journal of visualized experiments: JoVE (111). doi:10.3791/53431.
Publication
Reddyhoff D, Ward J, Williams D, et al. (2015). Timescale analysis of a mathematical model of acetaminophen metabolism and toxicity. Journal of theoretical biology 386: 132-146. doi:10.1016/j.jtbi.2015.08.021.
Challenge complete
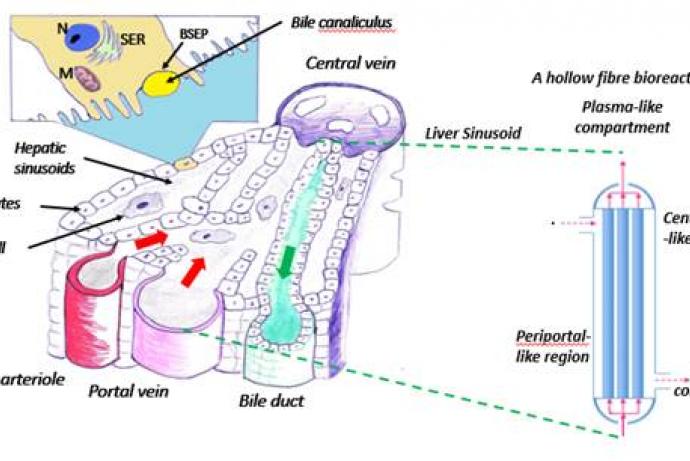
The multi-disciplinary team led by Dr Steven Webb, Liverpool John Moores University has developed a zonated hollow fibre bioreactor that more closely replicates the architecture and physiology of the liver for toxicology testing.
Review
Williams DP et al. (2012). Novel in vitro and mathematical models for the prediction of chemical toxicity.Toxicology Research. 2, 40. DOI: 10.1039/c2tx20031g
Challenge awarded
A team led by Dr Dominic Williams, University of Liverpool, has been awarded £997,634 to deliver the project: Development and mathematical modelling of zonated hollow fibre bioreactors for in vitro to in vivo extrapolation of systemic chemical toxicity.
Challenge launched
Sponsored by Unilever, Syngenta and AstraZeneca and co-funded by DEFRA, the IVIVE Challenge aims to develop a model that provides understanding of the relevance of toxicity concentration response data from human in vitro systems to predictions of safety following relevant in vivo human exposure.
Background
The safety assessment of new chemicals across the industrial chemical, agrochemical, pharmaceutical and consumer product sectors has long relied on high dose treatments in animals with default methods for extrapolating observed results to low level exposures in human populations. These traditional ‘whole-animal’ methods are expensive, can use many animals, and can sometimes be misleading with respect to human safety risk. As a result, increasing emphasis has centred on the development of predictive in vitro models for endpoints of toxicity, and their use to provide mode-of action understanding within the risk assessment process. Although progress has been made in developing in vitro models to predict some chemical toxicities such as skin irritation and corrosion, models to detect systemic toxicity across multiple organs are not currently available. Recently published opinions by the EU Scientific Committee on Consumer Safety (1) and a review by experts selected by the European Commission (2) indicate that, in the future, greater priority needs to be given to developing non-animal approaches which provide biological and chemical concentration-response data that can be integrated into consumer exposure and safety risk assessments. In 2007 the US National Research Council (NRC) issued its landmark report on “Toxicity Testing in the 21st Century: A Vision and a Strategy” (TT21C; (3)). The report sees a future in which routine toxicity testing would be conducted in human cells, human tissue surrogates, or human cell lines in vitro by evaluating cellular responses in a suite of toxicity pathway assays. These tools would enable risk assessors to predict regions of exposure that are expected to be without adverse consequences, rather than making predictions on the incidence of specific adverse responses in human populations. A key element to the realisation of this vision is the development of systems to understand exposure parameters in vitro and their extrapolation to inform safe in vivo exposure/in use scenarios. This will be the focus for this Challenge.
3Rs benefits
Historically, ‘alternative’ methods in toxicology have aimed to reproduce data generated using animal-based models. The aim of this Challenge is not to predict animal toxicity data but rather focus on safety risk assessment based on data relevant to human use as outlined in the TT21C vision. As such, if successful long term, the Challenge will ultimately provide tools and a means to address safety without use of animals.
Challenge winner
Project team led by:
Full Challenge information
A multi-disciplinary team led by Dr Steven Webb, Liverpool John Moores University, has developed a zonated hollow fibre bioreactor (HFB) that more closely replicates the architecture and physiology of the liver for toxicology testing (Storm et al., 2016). The system incorporates physiologically relevant 3D cell architecture, continuous fluid flow and oxygen gradients to promote liver zonation.
Mathematical modelling played a large role in the development of the HFB, for example, in predicting the optimal operating conditions of the HFB to best recapitulate the zonated liver physiology, and reduced development time by approximately six to twelve months. The exact design may not have been reached at all without the mathematical modelling.
The system is composed of a borosilicate glass module fitted with three plasma treated porous polystyrene fibres. Hepatocytes are seeded onto the outer fibre wall and the lumen of the fibre acts as the sinusoid (blood vessel) through which media is perfused. There is an oxygen gradient along the fibres to promote liver zonation. The junctions between the cells and the extracapillary space around the outside of the fibres then act as a bile canaliculi compartment.
The system has been optimised using the HepG2/C3A cell line and compared to 2D static monolayer cultures. Based on the analysis of multiple morphological and functional parameters, the HFB is an improved model system for HepG2/C3A cells (Storm et al., 2016).
The next steps are to further validate the system with primary heptocytes and a wider range of compounds, plus to manufacture an easy-to-use prototype device for user trial.
For further information about the HFB system please contact Dr Steven Webb.