NephroTube
The aim of this Challenge was to develop a multi-compartmental, microfluidic tissue assay to model the renal tubular injury observed in nephrotoxicity. The assay was to mimic the 3D architecture of the kidney tubules with microfluidics and chip arrays and possess the ability to reproduce the tubular response to known nephrotoxicants.
To address this Challenge, the team led by Dr Martijn Wilmer, Radboud University Medical Center, have developed 'NephroScreen', an in vitro organ-on-a-chip model consisting of perfused epithelial kidney tubules in OrganoPlates® as part of the MIMETAS product range.
This project and its impacts are featured as a case study in the 2019 CRACK IT Review.
10 years of CRACK IT webinar: Chipping away at in vivo nephrotoxicity testing

In June 2021, we held a webinar showcasing the NephroScreen human kidney-on-a-chip platform, developed by Mimetas to address the 2013 NephroTube CRACK IT Challenge, which could replace in vivo nephrotoxicity studies in drug development. Dr Linda Gijzen (Project Scientist, Mimetas) will describe how Challenge funding enabled the development, validation and wider uptake of the technology. The recording of the webinar is now available online, as part of our 10 years of CRACK IT celebrations.
Vormann MK, et al. (2021). Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies. Journal of Pharmaceutical Sciences. doi: 10.1016/j.xphs.2021.01.028
Publication
Vriend J, et al. (2019). Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochimica et Biophysica Acta (BBA) - General Subjects. doi.org/10.1016/j.bbagen.2019.129433.
Publication
Nieskens TT, et al. (2018). Expression of organic anion transporter 1 or 3 in human kidney proximal tubule cells reduces cisplatin sensitivity. Drug Metabolism and Disposition 1;46(5):592-9. doi:10.1124/dmd.117.079384.
Publications
A series of articles in a special topical collection in American Association of Pharmaceutical Scientists Journal:
Vormann M K, Gijzen L, Hutter S, et al. (2018). Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. AAPS J 20:90. doi:10.1208/s12248-018-0248-z.
Vriend J, Nieskens T T G, Vormann M K, et al. (2018). Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip. AAPS J 20:87. doi.org/10.1208/s12248-018-0247-0.
Suter-Dick L, Mauch L, Ramp D, et al. (2018). Combining Extracellular miRNA Determination with Microfluidic 3D Cell Cultures for the Assessment of Nephrotoxicity: a Proof of Concept Study. AAPS 20: 86. doi:10.1208/s12248-018-0245-2.
Conference presentation
Society of Toxicology 57th Annual Meeting (San Antonio, USA)
A Novel Predictive Model for Nephrotoxicity Based on Integration of a Multi-Parametric High-Content Biology Assay in ciPTEC-OAT1 and Drug Exposure.
Conference presentation
EuroTox (Bratislava, Slovakia)
Development of a high content assay in ciPTEC-OAT1 as an in vitro model to predict drug-induced nephrotoxicity.
Product launched
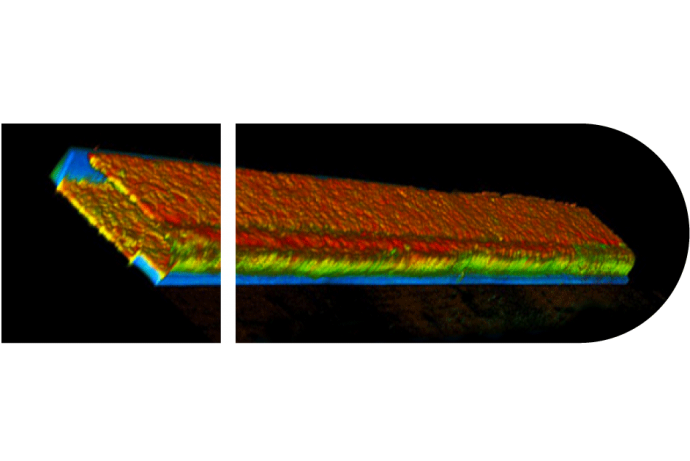
The Human Kidney Model uses a ECM gel in a three-channel OrganoPlate® to provide a physiologically-relevant 3D culture system.
Challenge completed
The NephroTube Challenge led by Dr Martijn Wilmer, Radboud University Medical Center, has been successfully completed. In the process, the team developed 'NephroScreen' an in vitro organ-on-a-chip model consisting of perfused epithelial kidney tubules in OrganoPlates® as part of the MIMETAS product range.
Conference presentation
Society of Toxicology 55th Annual Meeting (New Orleans, Louisiana)
Evaluation of the Human Renal Proximal Tubule Epithelial Cell Line, SA7K, in a Multiplexed qHTS Platform for Investigating Nephrotoxicity and a 3D Organ-on-a-Chip OrganoPlate™ Platform for Measuring Renal Clearance.
Publication
Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R (2016). Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends in biotechnology 1;34(2):156-70. doi:10.1016/j.tibtech.2015.11.001.
Publication
Nieskens TT, Peters JG, Schreurs MJ, Smits N, Woestenenk R, Jansen K, van der Made TK, Röring M, Hilgendorf C, Wilmer MJ, Masereeuw R (2016). A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. The AAPS journal 1;18(2):465-75. doi:10.1208/s12248-016-9871-8.
Phase 2 awarded
A team led by Dr Martijn Wilmer, Radboud University Medical Center, has been awarded £999,975 to deliver the project 'NephroScreen: toxicity screening with a human renal cell line in a perfused titerplate'.
Phase 1 awarded
Four Phase 1 Awards were made to project teams led by:
- Dr Colin Brown, Newcastle University, £99,726.
- Dr Paul Jennings, Medizinische Universität Innsbruck, £99,330.
- Dr Roisin Owens, Ecole Nationale Supérieure des Mines de St. Etienne, £100,000.
- Dr Martijn Wilmer, Radboud University Medical Center, £99,323.
Challenge launched
Sponsored by GSK, Pfizer and Roche, the NephroTube Challenge aims to develop a multi-compartmental, microfluidic tissue assay that models the renal tubular injury observed in nephrotoxicity. The assay should model the 3D architecture of the kidney tubules with microfluidics and chip arrays and possess the ability to reproduce the tubular response to known nephrotoxicant.
Background
Drug-induced organ toxicity accounts for 30% of all drugs that fail prior to reaching the market. Within this, nephrotoxicity accounts for 2% of failures in the preclinical stages and 19% of all failures in Phase III. There is a significant translational gap between the preclinical models of nephrotoxicity and their predictive value through the clinical stages of drug development. Current cell-based models can provide valuable information but do not accurately predict toxicity in humans. A more complex, human tissue-based, in vitro model that can accurately measure toxic effects would reduce the failure rates from preclinical to clinical stages of development by providing a more relevant model.
A Challenge to develop predictive in vitro screens for nephrotoxicity was featured in the CRACK IT 2011 Competition. No awards were made and the Challenge has now been amended to capitalise on novel microfluidic technologies that have recently become available and focus on human tissue systems rather than multiple preclinical species.
3Rs benefits
- A typical investigative animal study to assess nephrotoxicity would use at least 26 rodents (and substantially more animals if both sexes were necessary). New in vitro technologies that replace the need for animal use in toxicity testing could significantly reduce these numbers.
- Use of predictive human systems in preclinical toxicology testing will reduce the drug attrition that results from poor translation from preclinical studies into humans.
- Where animals are still used, information on the underlying mechanisms of toxicity will help refine study designs including dosing regimes and species selection.
Phase 1 winners
Project teams led by:
- Dr Colin Brown, Newcastle University, £99,726.
- Dr Paul Jennings, Medizinische Universität Innsbruck, £99,330.
- Dr Roisin Owens, Ecole Nationale Supérieure des Mines de St. Etienne, £100,000.
- Dr Martijn Wilmer, Radboud University Medical Center, £99,323.
Phase 2 winner
Project team led by:
Full Challenge information
The NephroTube team led by Dr Martijn Wilmer has developed a microfluidic kidney proximal tubule on-a-chip device that can be used as an early screen to detect drug-induced nephrotoxicity, reducing the number of animal studies carried out. The device is high throughput (up to 96 kidney proximal tubules/plate), easy to use (does not require complex pumps) and affordable, making it suitable for routine drug screening.
Kidney proximal tubule epithelial cells are grown against an extracellular matrix gel in OrganoPlates® (MIMETAS) and polarised 3D tubules are formed under fluid flow. Multiple endpoints/biomarkers that reflect drug-induced nephrotoxicity can be measured from the device e.g. drug transporter function, barrier integrity, cell viability etc.
The model has been characterised using two different proximal tubule cell lines (ciPTEC and Sigma RPTEC) and validated with nephrotoxic compounds provided by the Challenge Sponsors. Reproducibility has been demonstrated between laboratories, confirming the robustness of the device. Standard Operating Procedures have been developed for multiple endpoints and the team held a hands-on workshop to facilitate technology transfer to the end-user.
To access the platform, please contact MIMETAS in the first instance.
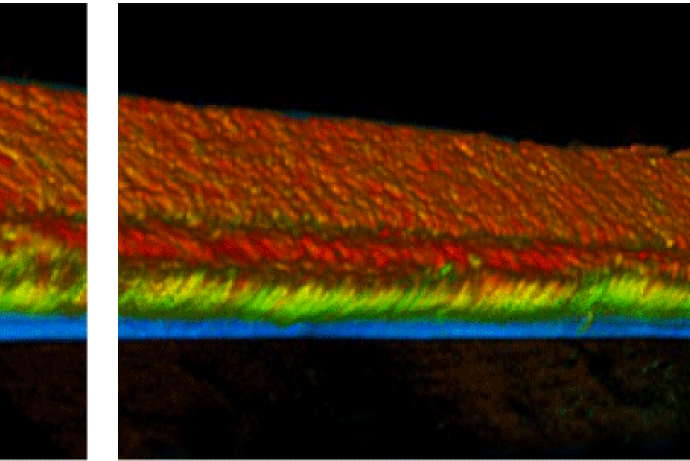
NephroScreen: a human kidney model for drug-induced nephrotoxicity screening
Current 2D cell culture and animal models are insufficient to correctly predict drug effects on human kidney function. While 2D culture systems fail to differentiate and express transporters at physiological levels, there is a major translation gap between animal models and humans. MIMETAS have developed perfused epithelial kidney tubules in OrganoPlates® by seeding renal proximal tubule cells against an extracellular matrix (ECM) gel. Upon initiation of a perfusion flow, a tubular structure is formed. These kidney tubules can be derived from a variety of cell sources and are fully differentiated and can be used to replace the use of animal models for a variety of basic and applied studies of the kidney.
MIMETAS is a global leader in tissue and disease modeling using organ-on-a-chip technology. Its proprietary OrganoPlate® platform contains up to 96 tissue culture chips and enables perfused 3D (co-)culture in a membrane-free manner. The platform supports the development and high-throughput screening of physiologically relevant healthy and disease models.
NephroScreen – modeling renal tubular injury in vitro
A perfused epithelial kidney model developed in a three-lane OrganoPlate® that can be used as an early in vitro screen for drug-induced nephrotoxicity. The OrganoPlate® platform does not require complex pumps, consists of 40 tissue culture chips in parallel with apical and basal access, and is based on a 385 well-plate it makes the platform suitable for high-throughput and routine drug screening. NephroScreen has been independently characterized by three different laboratories using two proximal tubule human cell lines and validated with 12 nephrotoxic compounds provided by pharmaceutical companies. The model can accurately detect toxicities when compared with preclinical and/or clinical data and can be used to replace the use of animal models for a variety of basic and applied studies of the kidney.
Features



Access the technology
Find out more about this product on the MIMETAS website.
References
Vormann MK et al. (2021). Implementation of a Human Renal Proximal Tubule on a Chip for Nephrotoxicity and Drug Interaction Studies. Journal of Pharmaceutical Sciences 110(4): 1601-1614. doi: 10.1016/j.xphs.2021.01.028
Vormann MK et al. (2018). Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. The AAPS Journal 20(90). doi: 10.1208/s12248-018-0248-z
Vriend J et al. (2018). Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip. The AAPS Journal 20(5):87. doi: 10.1208/s12248-018-0247-0
Suter-Dick L et al. (2018). Combining Extracellular miRNA Determination with Microfluidic 3D Cell Cultures for the Assessment of Nephrotoxicity: a Proof of Concept Study. The AAPS Journal 20(86). doi: 10.1208/s12248-018-0245-2