QSARs Mix
The aim of this Challenge was to use existing toxicological data repositories to develop in silico tools which could be used to predict toxicology endpoints for substances of interest in order to waive in vivo studies. The developed tools would be of benefit to any company that imports or manufactures chemicals. The tools would also be of use to any company seeking to bridge data gaps in skin and eye irritation.
To address this Challenge, the team at KREATiS, led by Dr Paul Thomas, has developed iSafeRabbit, a set of high accuracy QSARs for predicting the skin and eye irritation and corrosivity potential of petrochemical substances and mixtures, to replace in vivo animal studies.
Publication
Charmeau-Genevois C et al. (2021). A simplified index to quantify the irritation/corrosion potential of chemicals – Part 2: Eye. Regulatory Toxicology and Pharmacology 123, 104935. doi.org/10.1016/j.yrtph.2021.104935
Publication
Charmeau-Genevois C et al. (2021). A simplified index to quantify the irritation/corrosion potential of chemicals – Part I: Skin. Regulatory Toxicology and Pharmacology. 123, 104922. doi.org/10.1016/j.yrtph.2021.104922
Updated product

The iSafeRat skin/eye irritation/corrosion prediction model v2.0 is a new updated set of high accuracy QSARs, formerly known as iSafeRabbit v1.1.
Training Course
Replacing experimentation using Read-across and QSAR modelling.
CRACK IT Solution
Through CRACK IT Solutions, KREATiS is looking to extend the scope of iSafeRabbit and validate the model to include new chemical groups.
Presentation at the 2016 CRACK IT Challenge launch event
Dr Paul Thomas presented the QSARs Mix Challenge at the 2016 CRACK IT Challenge launch event.
Conference presentation
EUROTOX 2016 (Brussels, Belgium)
iSafeRabbit QSAR To Predict Skin And Eye Irritation Potency Of Organic Chemicals.
NC3Rs workshop
Applying pathways-based approaches across the biosciences (London, UK)
A High Accuracy QSAR based on rabbit data to predict the human skin irritation potential of individual constituents and mixtures.
Product launched

A High Accuracy QSAR based on rabbit data to predict the human skin irritation potential of individual constituents and mixtures.
Challenge completed
The QSARs Mix CRACK IT Challenge led by Dr Paul Thomas, KREATiS, has been successfully completed. In the process, the team developed iSafeRabbit, a set of high accuracy QSARs for predicting the skin and eye irritation and corrosivity potential of petrochemical substances and mixtures, to replace in vivo animal studies.
Challenge awarded
A team led by Dr Paul Thomas, KREATiS and Dr Peter Jenkinson, CEHTRA, have been awarded £96,500 to deliver the project: Development of high accuracy QSARs for mixtures to bridge the data gaps in skin and eye irritation.
Challenge launched
Sponsored by Shell, the QSARs Mix Challenge aims to develop a more predictive and relevant tool to quantitatively predict the skin and eye irritation of chemical mixtures, without the use of animals.
Background
Where substances are manufactured in quantities of over ten tonnes per year, the European REACH regulation requires specific toxicological information for the substance to be registered. Similar toxicological information is also required for registering substances in other regions of the world. Companies also routinely conduct toxicology testing for candidate selection during product development. In one area of mandatory toxicity testing, the prediction skin and eye irritation effects, investigators may utilise in vitro models for prioritisation of candidates and potential classification, however in vivo models may be required for classification of substances. Such animal tests can only be avoided with strong scientific justification. The use of in silico prediction methods such as (Quantitative) Structure Activity Relationships ((Q)SARs) and expert systems are starting to build a toolbox for providing scientific justification, but more development is needed to provide adequate information to waive the in vivo studies.
(Q)SARs and expert systems for human health endpoints are based on the assumption that the toxicity of a compound is related to its chemical structure. (Q)SARs must meet quality standards and be scientifically validated according to the OECD principles. A 2006 European Commission report concluded ‘that the further development, validation and documentation of in silico systems for local toxicity to the skin and eye are necessary’. Expert systems are sets of ‘if-then’ rules or criteria to classify a chemical into various categories (e.g. eye irritant). Currently, these rules are defined by physical and chemical property thresholds. A more promising way forward is the use of structural alerts. These are sets of sub-structures that are identified in a molecular formula; an occurrence of any of these triggers the alert (e.g. skin or eye irritant). It is the sub-structures within a substance that dictate its physical properties, therefore it is important to correlate toxicity directly with these structures rather than the physical chemical parameters that may not always be available.
The current (Q)SAR/ structural alert tools are limited in the data sources used to develop them and are not amenable to mixtures. A large proportion of substances imported or manufactured in the EU are mixtures. The development of computational models that can be used to interrogate mixtures of chemicals would present a significant innovation in this area, broaden their application and reduce animal use.
The aim of this Challenge is that a model or expert system be created which allows for the reliable prediction of skin and eye irritation based on structural information or ‘structural alerts’. Specifically, this tool should be able to predict the toxicity associated with a mixture of substances assuming a proper compositional analysis of the test mixture and applying a weighted average score approach.
If successful these tools will:
- Provide a more predictive and relevant tool-set to predict potential toxicity related to skin and eye irritation that can be used to reduce in vivo studies.
- Enable rapid screening of potential candidates.
- Decrease development costs and time-to-market.
- With further development, the approach could be translated to other toxicity endpoints such as reproductive and developmental toxicity.
3Rs benefits
- Companies performing in vivo studies for skin and eye irritation for registration of new substances can utilise >250 rabbits per year.
- These irritation studies are invasive, further supporting replacement of the in vivo models.
The proposed model will improve the predictive capacity of the current in silico models, permitting the early identification of potential toxicities in candidate selection without having to use in vivo studies and contribute to the scientific justification to waive the in vivo studies for skin and eye irritation for those taken forward to registration.
Single Phase Challenge winner
Project team led by:
Full Challenge information
KREATiS, in collaboration with Challenge Sponsors Shell, has developed iSafeRabbit, a set of high accuracy QSARs for predicting the skin and eye irritation and corrosivity potential of petrochemical substances and mixtures, to replace in vivo animal studies.
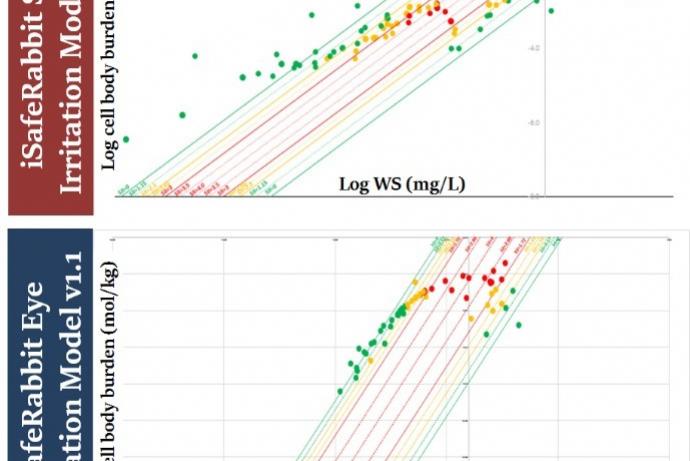
The iSafeRabbit skin/eye models have been validated for 12 chemical families and their predictions have a true positive/negative rate of 95% using validated in vivo experimental data as reference. The models also satisfy the five recommended OECD principles for QSAR models making them fit for regulatory purposes.
To maximise its application and wide-scale use as an in silico alternative to in vivo experimentation, KREATiS was looking to extend the scope of iSafeRabbit and validate the model to include new chemical groups. KREATiS was seeking in vitro and in vivo irritation data from various industrial domains, in particular cosmetics and household products, but also data on active ingredients from pharmaceuticals, pesticides and biocides.
To find out more about iSafeRabbit visit the KREATiS website or contact the KREATiS team.
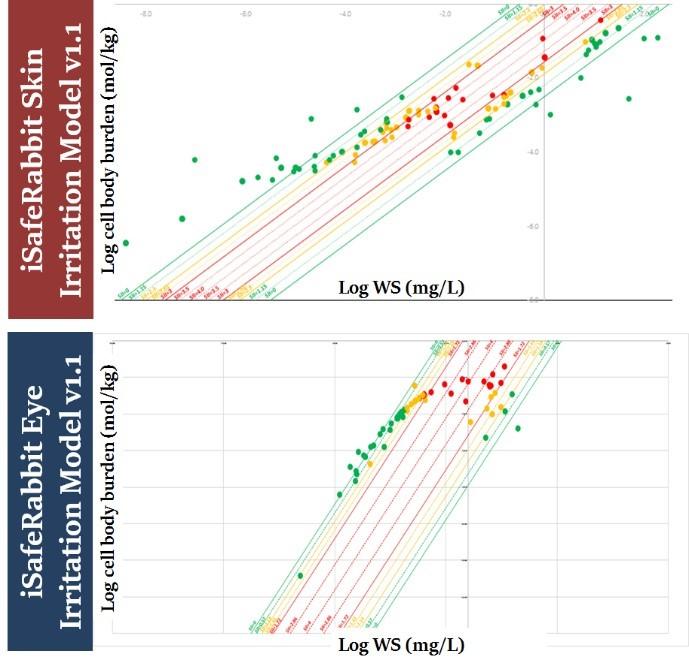
iSafeRabbit skin and eye irriation QSARs
iSafeRat® Skin/Eye Irritation/Corrosion Prediction Model
iSafeRat skin/eye irritation/corrosion prediction model v2.0, formerly known as iSafeRabbit v1.1, is a set of high accuracy QSARs (HA-QSARs) for predicting the skin and eye irritation and corrosivity potential of chemical substances that aims to replace in vivo animal studies. The goal was also to fill the gap where in vitro studies currently fail to detect irritation to eyes but either detect corrosives or non-irritants.
The models outputs are the result of quantitative predictions of cell burdens of the test substance calculated to be accumulated in the skin and eye tissue. These cell burdens depend exclusively upon the intrinsic physico-chemical properties of the test substance. These mechanistic models are able to provide predictions for the skin/eye irritation potential of the chemical substances that are equivalent to the UN GHS classification system: Category 1 (corrosives), Category 2 (Irritants) and No-category (NC, i.e., non-irritants), making this alternative method unique in discerning Category 2 chemicals for eye irritation.
Access the technology
Find out more about this product and access the model on the Kreatis website.