Registration Details
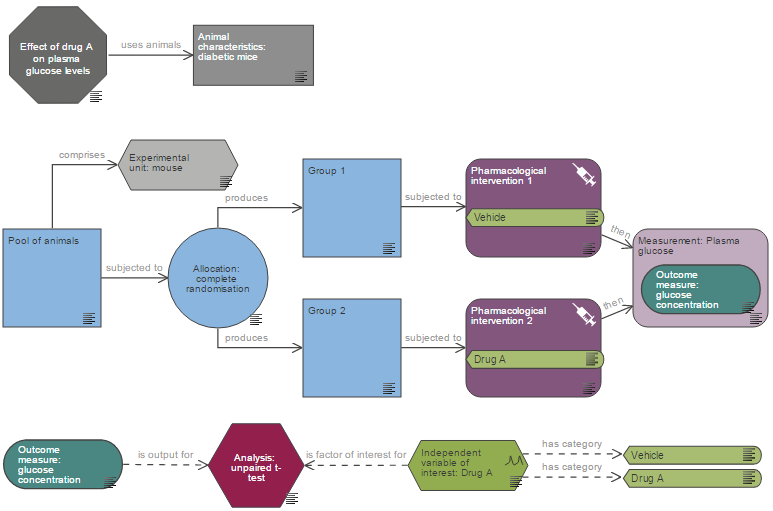
The Experimental Design Assistant (EDA) is a free online software that helps researchers design robust in vivo experiments that are more likely to give reproducible results. This interactive virtual workshop on using the EDA will be helpful for anyone involved in planning and designing experiments or teaching experimental design.
During the session Dr Esther Pearl (NC3Rs Programme Manager for Experimental Design) will give a live demonstration of the EDA before answering your questions and providing personalised feedback as you design your own experiment.
By the end of the session you will be able to use the EDA to:
- Design your next experiment.
- Receive tailored feedback on your experimental plans.
- Identify statistical analysis methods relevant to your design.
- Determine an appropriate sample size.
- Create a randomisation sequence.
- Communicate your plans clearly with others, including with colleagues, collaborators, grant and ethical review bodies, and publishers.
Registration information
To enable an interactive session, the session will be held as a Zoom meeting (rather than a webinar) and attendee numbers will be limited on a first-come, first-served basis. If you are unable to attend this demonstration, other dates and times will be available soon. Registration closes on Monday 11 November.
You can earn one CPD point attending an EDA demonstration. Certificates are available on request only to registered participants attending through their unique Zoom link.
Further resources
For further information on good experimental design visit the Experimental Design pages of the EDA website, or watch the recording of our webinar on Best Practice in Experimental Design.
You can learn about the importance of reporting experiments thoroughly by visiting the ARRIVE guidelines website, or watching the recording of our webinar on the ARRIVE guidelines 2.0.
