An overview of our toxicology and regulatory sciences programme.

An overview of our toxicology and regulatory sciences programme.

Outcomes from the SAFE CRACK IT Challenge to replace fish studies in chemical safety screening and environmental risk assessment.
Quarterly e-newsletter to keep the scientific community updated on news from the NC3Rs Toxicology and Regulatory Sciences programme.

Expert perspectives on AI approaches to improve toxicity prediction and reduce reliance on animal use in regulatory science.
Join our community of researchers, developers, industry and regulatory end-users working together to accelerate the use of new approach methodologies.

Supporting the uptake of new approach methodologies to reduce reliance on traditional animal toxicity tests.

Winning and highly commended researchers share their work to strengthen the evidence base for replacement technologies at the 3Rs Prize ceremony.

Using exposure science to refine and reduce animal use, and support the uptake of new approach methodologies
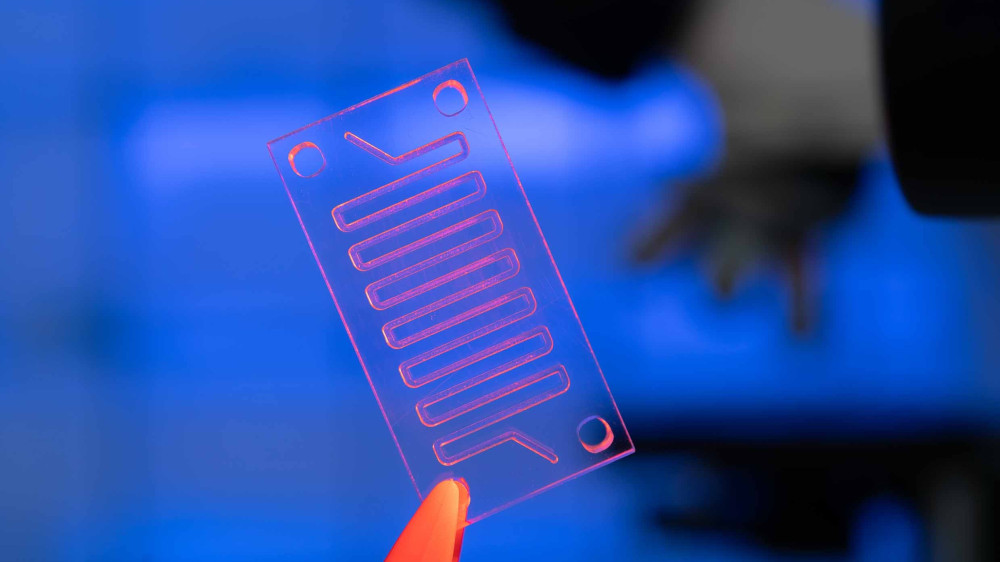
Learn about the winning in vitro method for evaluating hormone potency and the highly commended organ-on-a-chip device to determine liver toxicity.
Projects to ensure that the in vivo tests involved in ED assessment are as robust as possible, particularly focusing on aquatic species.

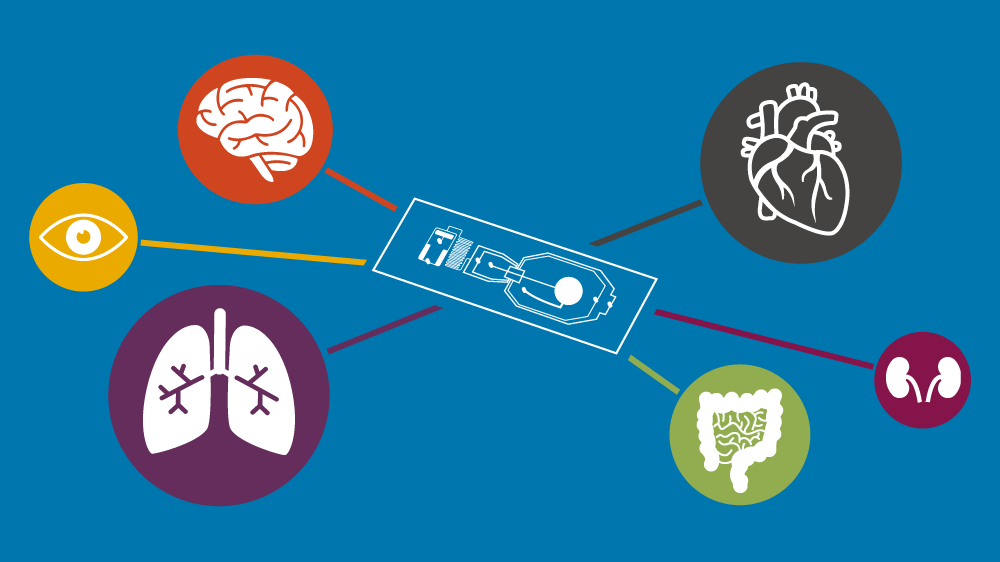
Resources and activities around NAMs (replacement technologies used to assess drug and chemical safety).
A set of resources to support the adoption of the 3Rs in quality control and batch release testing of vaccines and biological therapeutics
Hear about the Prize-winning animal-free tonsil organoid model and the first ever in silico trial for a medical device which was highly commended.

Information and support for the adoption of alternative approaches and technologies.
Key results and recommendations from an EPAA/MEB/NC3Rs collaboration focused on chronic toxicity studies for monoclonal antibodies (mAbs).

A webinar to highlight available opportunities to minimising non-human primate use in drug development.

Opportunities to reduce the use of non-human primates in toxicology programmes.

A series of workshops as part of our project to implement the 3Rs in WHO guidelines.
Webinar showcasing ongoing and novel initiatives that replace, reduce and/or refine fish acute toxicity studies.

A virtual workshop to raise awareness of currently available New Approach Methodologies (NAMs) and overcome barriers to implementation.

Reducing and refining acute toxicity tests in the pharmaceutical and chemicals industries.
Providing an evidence base to refine and reduce the use of vertebrates in (regulatory) ecotoxicology studies

Guidance on reducing or refining animal use for studies with cardiovascular, central nervous system and respiratory functional measurements.

Projects providing guidance to reduce animal use or refine procedures within toxicology studies.