Anaesthesia
On this page
Introduction
Anaesthetising laboratory animals is a significant refinement of research procedures, since it results in a lack of awareness of any potentially painful or distressing events. Anaesthesia can be used to prevent pain during potentially painful procedures, such as surgery, or to provide a humane means of immobilising an animal for procedures, such as imaging.
When selecting a method of anaesthesia, it is important to consider:
- The required depth and duration of anaesthesia
- Whether the method selected will cause the minimum of interference with the purpose of the research procedure
In addition the method of anaesthesia should ideally be:
- Simple to administer, without causing significant distress to the animal
- Free from undesirable side effects and allow a smooth and uncomplicated recovery
In broad terms, the available methods fall into two groups; inhalational anaesthetics and those administered by injection. Although selecting a single agent may seem a good way of minimising the potential interactions with research, the effects of all anaesthetic agents are dose-dependent. To reduce these potentially undesirable side-effects, use of a combination of agents, at lower dose rates, is often advantageous. This approach of “balanced anaesthesia” is often simple to implement. For example, administration of an opioid analgesic, such as buprenorphine, 20-30 minutes before induction of anaesthesia with an inhalational anaesthetic, such as isoflurane, enables the concentration of isoflurane needed to maintain anaesthesia to be reduced by around 0.5%, with a consequent reduction in the degree of respiratory and cardiovascular depression produced by this anaesthetic agent.
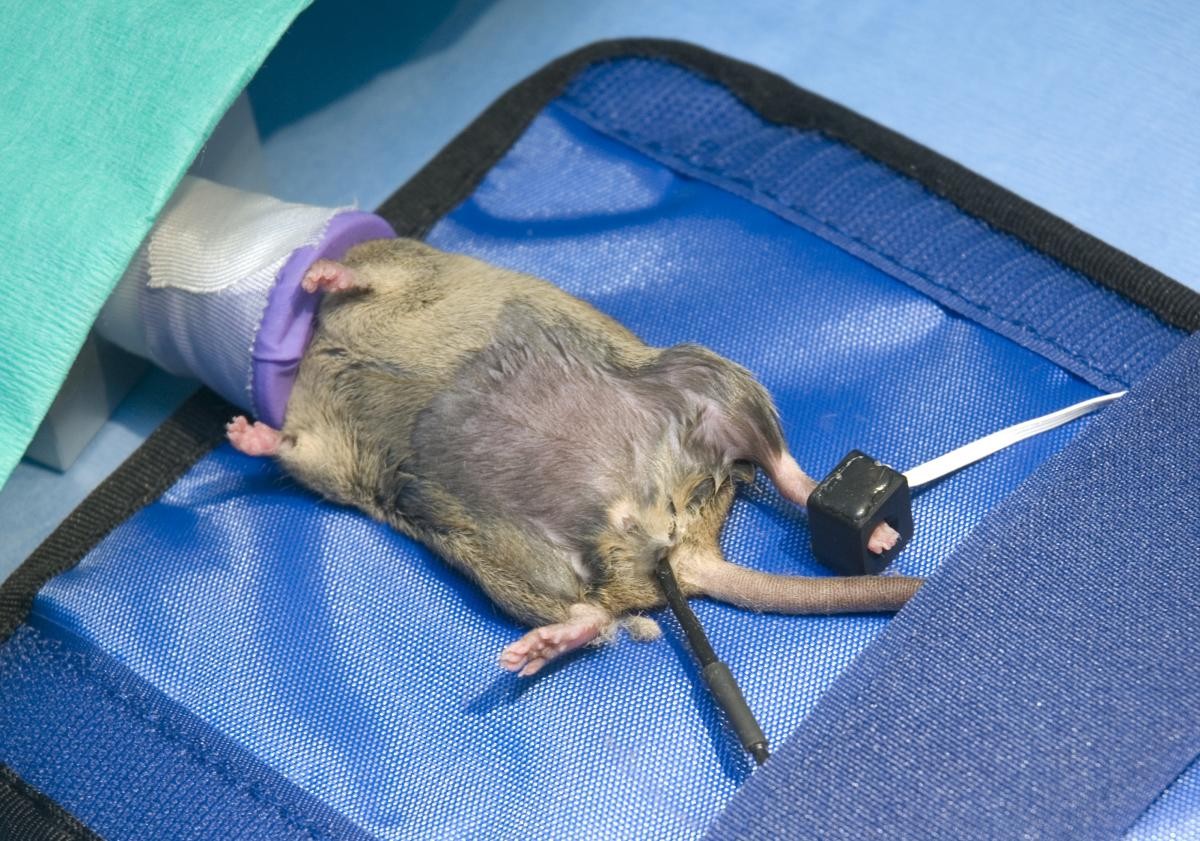
When anaesthetising animals for relatively short procedures, simple clinical monitoring of the animal by an assistant may be sufficient. Depth of anaesthesia can be assessed by testing withdrawal reflexes, and the pattern and depth of respiration and colour of mucus membranes can be evaluated. In larger species, palpating a peripheral pulse can provide information about the degree of cardiovascular depression. However, clinical monitoring has some major limitations; for example it is not possible to count fast enough to assess the heart rate of a mouse. Considerable advances have been made in the development of electronic monitoring devices, and these are now available for both large and small laboratory animals. These devices are particularly useful when anaesthesia is more prolonged.
In addition to use of sophisticated devices, simple measures such as ensuring maintenance of body temperature, provision of oxygen and protecting the cornea from damage by use of protective eye ointment, all contribute both to refinement of anaesthesia and to the quality of research data that will be obtained.
Information on potentially suitable anaesthetic regimens can be obtained from a variety of sources, including the Named Veterinary Surgeon, and specialist laboratory animal anaesthesia and general veterinary anaesthesia textbooks. Obtaining information on potential interactions with research procedures is less easy to obtain and frequently requires a careful literature search. It is important to note that the agents available and the techniques used continually change, and simply selecting the method used in previous scientific publications in the same field does not guarantee that the most appropriate method will be selected.
E-learning modules
The NC3Rs has funded a series of e-learning modules on anaesthesia, developed by FLAIRE Learning and Newcastle University, that are freely available for training and continuing professional development. The modules can be incorporated into Home Office Personal Licensee (PIL) Category C training.
Ensuring best practice in anaesthesia for minor procedures.
Factors to consider prior to anaesthesia of laboratory animals.
How to choose appropriate anaesthetic agents and regimens.
Why and how to monitor anaesthetised animals.
Use of different anaesthetic breathing systems, airway management, and neuromuscular blocking drugs.
Managing anaesthesia and what can be done to prevent problems.
Recovery from anaesthesia and post-anaesthetic support.
Related resources
- Vets On Line Email (VOLE) - Email discussion list used by many Named Veterinary Surgeons (NVSs) under the Animals (Scientific Procedures) Act 1986. Contact: secretary@lava-vet.org.
References
- Flecknell (2015) Laboratory Animal Anaesthesia, 4th edition, Elsevier.
- ILAR, DELS, National Research Council (2009) Recognition and alleviation of pain in laboratory animals, The National Academies Press.
