Registration Details
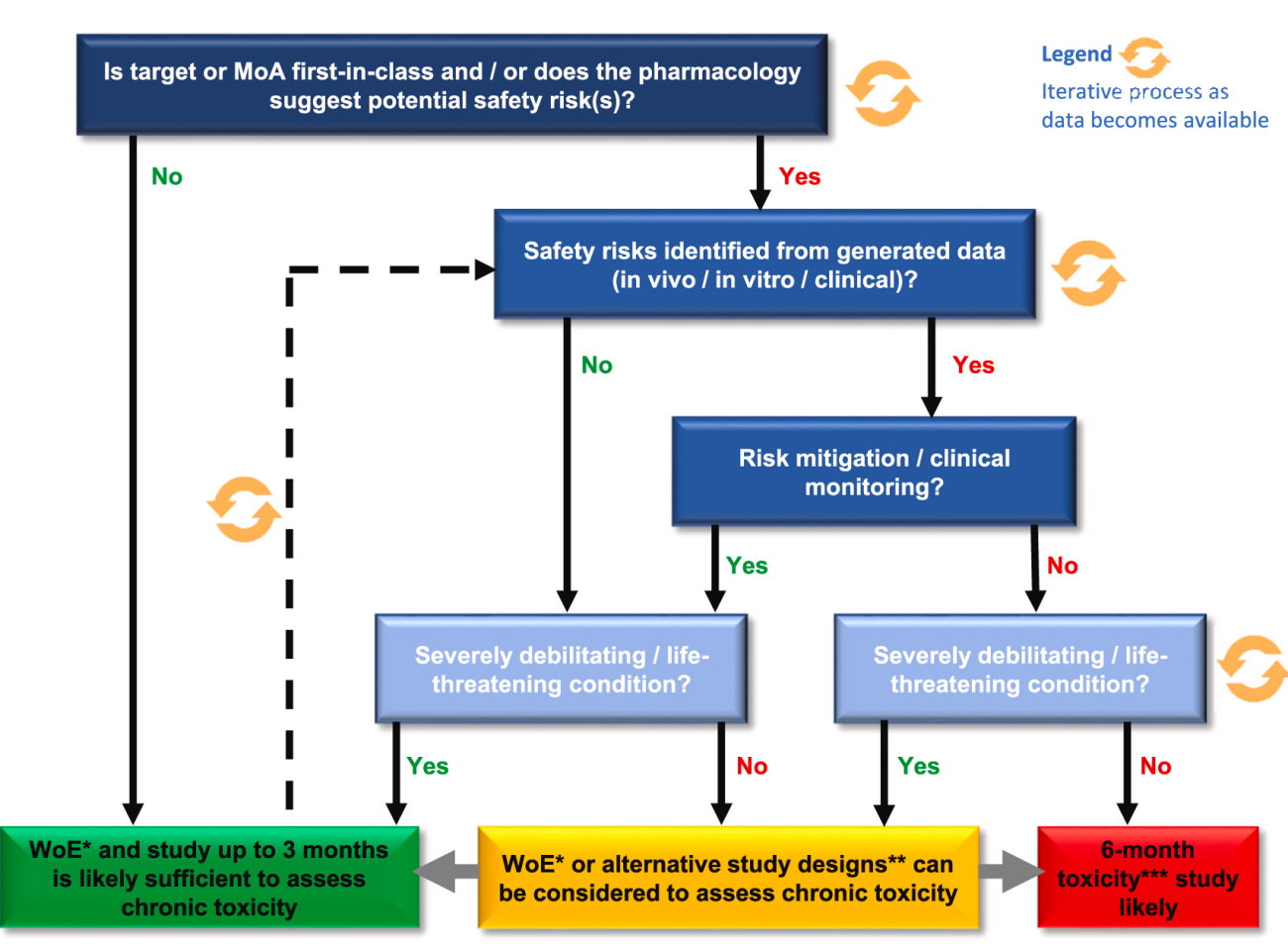
To support registration of monoclonal antibodies (mAbs) for chronic indications, 6-month toxicity studies have historically been conducted as per ICH S6(R1) guidance. Experience with mAb development has shown a relatively benign and well-understood safety profile for this class, with most toxicity findings anticipated based on pharmacology.
Under a European Partnership for Alternative Approaches to Animal Testing (EPAA) supported and funded project, a consortium of 14 pharmaceutical companies, the Medicines Evaluation Board (MEB) and the NC3Rs conducted a study to evaluate whether a 6-month toxicity study is still necessary to assess the long-term safety of mAbs.

Two recent publications from the project will be discussed during the webinar.
The aim of this joint NC3Rs/EPAA webinar is to provide an overview of the two recent publications from the project, discussing key results and recommendations. In particular, a proposed weight-of-evidence model will be highlighted, with case-studies explaining the potential decision processes. Additionally, 3Rs opportunities identified within the dataset, including group sizes and use of recovery animals, will be presented.
This webinar is relevant for regulatory and industry scientists working with mAbs and other drug modalities interested in exploring new approaches for chronic toxicity studies and opportunities to apply the 3Rs. There will be a panel discussion at the end of the webinar when audience members will be able to ask questions.
The agenda for the webinar is below:
| Time | Agenda Item |
|---|---|
| 14.00 – 14.10 |
Welcome and brief background to the project Lucinda Weir, GSK, UK (CHAIR) |
| 14.10 – 14.50 |
Re-evaluating the need for chronic toxicity studies with therapeutic monoclonal antibodies, using a weight of evidence approach Peter van Meer, Medicines Evaluation Board, The Netherlands |
| 14.50 – 15.10 |
Recovery animal use and other 3Rs opportunities in nonclinical safety assessment studies with monoclonal antibodies Helen Prior, NC3Rs, UK |
| 15.10 – 15.30 | Panel discussion and audience Q&A |
| 15.30 – 15.35 |
Concluding remarks Lucinda Weir, GSK, UK |
A recording of this webinar will be made available to delegates after the meeting.
Certificates of attendance are available on request only to registered participants attending through their unique Zoom link.
Event image: Fig 5, modified from Chien et al. 2023.
