Applying bioprinting approaches to improve drug and chemical product development

A report in Biofabrication authored by scientists from the Universities of Manchester and Strathclyde, AstraZeneca, Syngenta, Unilever and the NC3Rs highlights recommendations for applying bioprinting technology to improve safety testing while replacing the use of animal models.
Lead author, Dr Anthony Holmes, Head of technology development at the NC3Rs, said: “The development and application of human tissue-based models represents the future for safety and efficacy testing across a range of industries. The ability to print complex 3D structures using human cells which can be incorporated in organ-on-chip and novel bioreactor technologies puts bioprinting at the forefront of these developments. The UK has world-leading research in this area and companies, large and small, with the ability to take advantage of the new commercial opportunities. This is offered by improved decision-making tools that have the potential to result in more rapid discovery and development of medicines, agrichemicals, chemicals and consumer products, without using animals.”
3D printing has provided exciting opportunities in the mass manufacture of a wide variety of plastic and metal objects. Its application to the life sciences, however, has the potential to be transformative. Once solely in the realms of science fiction, 3D bioprinting is now very much a reality. Specially modified printers artificially construct living tissue by printing layer upon layer of living cells to form 3D tissue and organ structures. These biomimetic tissue structures can be rapidly, accurately and reproducibly created at scale and with human cells. This makes them ideal tools for generating human relevant data in efficacy and safety studies as well as reducing the over-reliance on current animal and simple 2D in vitro models (see image) which have contributed to the high drug attrition rates seen across the pharmaceutical industry.
While interest in bioprinting is growing in the academic sector, its use in industry is as yet relatively limited. The report outlines future opportunities to use bioprinting in three industrial sectors – pharmaceutical, agrochemical and consumer products – focusing on three different applications:
- In vitro models of liver structure and function for assessment of hepatotoxicity and carcinogenicity in plant protection product development.
- Human-relevant models of respiratory disease in pharmaceutical drug development.
- Better models to assess the benefits and safety risks of consumer products on skin.
The importance of collaboration between stakeholders across academia, the SME sector and major industries in accelerating the wider uptake of bioprinting is highlighted throughout the report in terms of:
- Understanding how bioprinted models could be used by industry and regulators for decision making so that the tissues and organs generated are fit-for-purpose.
- Defining the strategies necessary for validating bioprinted models to inspire confidence in their utility.
- Recognising the multidisciplinary nature of bioprinting and the need to nurture long-term research partnerships.
- Combining bioprinting with other technological innovations such as microfluidic organ-on-chip platforms to maximise applications.
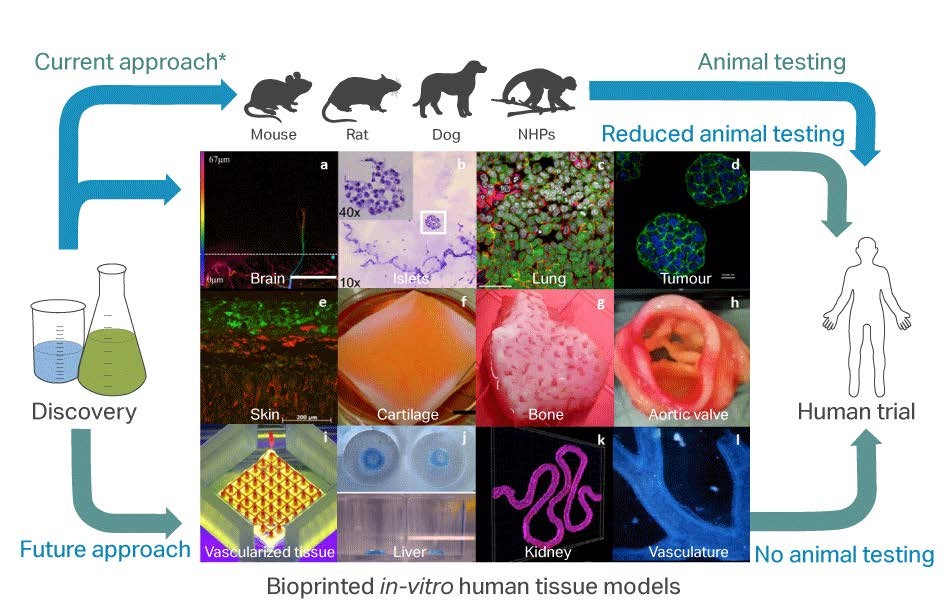
Application of bioprinting technologies to potentially improve drug and chemical development processes (* this does not apply to personal care/cosmetic products). Panels a-l provide as summary of some of the available 3D bioprinted in vitro human tissue models.
