Benchtop method for fabricating organ-on-a-chip devices wins International 3Rs Prize

An alternative technology making organ-on-a-chip systems more accessible to labs worldwide has won the 3Rs Prize, awarded by NC3Rs and co-funded by GSK.
In this article
Winner
Congratulations to Dr Daniel Ferreira at the Instituto de Investigação e Inovação em Saúde, Portugal who has been awarded the International 3Rs Prize for his publication in Advanced Science. Daniel and colleagues have adapted a method to enable organ-on-a-chip systems to be designed, developed and printed with commercially available equipment and materials.
Ferreira DA et al. (2021). Alternative to Soft Lithography for the Fabrication of Organ-on-a-Chip Elastomeric-Based Devices and Microactuators. Advanced Science 8:2003273. doi: 10.1002/advs.202003273
Organ-on-a-chip systems are a promising tool for reducing the reliance on animal models in basic and applied laboratory research. The devices consist of cells and/or tissue grown in microfluidic chips, which are composed of a clear flexible polymer. The chips are designed to closely mimic human physiology of a specific organ and can be applied in many research fields including disease modelling, toxicology and safety studies, and personalised medicine. Multiple chips, replicating different organs, can also be connected for organ-organ communication studies, and researching the interface between specific cells and tissues.
While organ-on-a-chip systems hold a lot of promise, there have been barriers to uptake including the cost and time to manufacture the devices, which are often bespoke. In his publication, Daniel demonstrates how commercially available sheets of thin polymer can be used with a cutting plotter to construct each layer of a microfluidic chip. The chip can then be assembled on a glass microscopic slide.
To better replicate human physiology, organ-on-a-chip systems often integrate membranes, Daniel describes how these can also be incorporated using the method featured in his publication. For example, one method to enhance the functional response of cultured hepatocytes is to flow cell media continually over a PET membrane above the cells, which promotes undisturbed fluid flow. The fabrication method featured in Daniel’s publication allows a PET membrane to be added, using bench-top laboratory equipment in a number of hours. These chips are stable at physiological temperature and humidity enabling in vitro toxicity testing.

Daniel also describes how biomechanical cues can be included within his method for chip assembly. When a vacuum is applied to a microactuator chamber a flexible membrane in the chip stretches expanding the substrate the cells are cultured on with resulting surface expansion equivalent to that within the gut, lung and heart. Daniel validated using a chip with an integrated microactuator by culturing a human gastric epithelial cell line using a stretching pattern designed to mimic peristalsis. Cells grown in these conditions had an average epithelial height equivalent to that observed in normal stomach epithelium and expressed Mucin-1 in a similar pattern to normal gastric mucosa. These qualities surpassed those of cells grown in static conditions and those grown with flow alone.
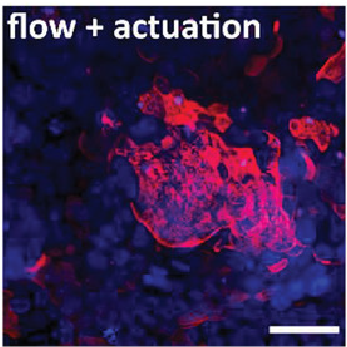
The method described in the Prize-winning publication enables organ-on-a-chip prototypes to be rapidly designed and assembled. The modularity of chip fabrication allows each aspect of the chip, such as robustness, cell culture maintenance and mechanical stretching, to be independently tested before new layers are added. Any alterations to the chip design can then be implemented and tested rapidly. Daniel will now use the Prize money to begin his independent academic career establishing a microfabrication lab. He will design an organ-on-a-chip platform as a proof-in-principle to study the molecular and cellular mechanisms that initiate hereditary diffuse gastric cancer using patient derived induced pluripotent stem cells.
The applications for the International 3Rs Prize are always of an extremely high standard and this year was no exception. The wealth of 3Rs research taking place in labs worldwide is astonishing and the Panel faced a difficult decision selecting a winner. I am excited to see the potential of the winning paper realised over the coming years, enabling easier access to this important 3Rs method.
Professor Kevin Shakesheff, Chair of the NC3Rs Board and 3Rs Prize Panel
In GSK, we are uniting science, technology and talent to get ahead of disease together. Our ambition over the next 10 years, is to positively impact the health of more than 2.5 billion people. Our strategy is centred around preventing and treating disease, focusing on the science and operating responsibly. We recognise that whilst our technological advances mean that much of our research can be done using non-animal methods, studies in animals are still needed. GSK's commitment to the International 3Rs Prize reflects how our ambition of replacing, reducing and refining the use of animals is integral to our purpose of discovering and developing transformational medicines and vaccines.
Dr Rehana Sidat, VP Quality and Risk Management, Research, GSK.
Highly commended
Dr Ben Newland at Cardiff University has also been highly commended for his publication describing a method to induce focal lesions in tissue slices to create an ex vivo model of multiple sclerosis (MS). MS is modelled in rodents by immunising against myelin, inducing experimental autoimmune encephalomyelitis, which results in myelin damage similar to patients with MS. These studies are associated with a high level of suffering and the need for specialist husbandry and care.
Eigel D et al. (2019). Cryogel scaffolds for regionally constrained delivery of lysophosphatidylcholine to central nervous system slice cultures: A model of focal demyelination for multiple sclerosis research. Acta Biomaterialia 97:216. doi: 10.1016/j.actbio.2019.08.030
Ex vivo slice culture methods have previously been developed but these induce demyelination across the full tissue, which is not representative of how MS develops in patients. In his publication, Ben describes a method using cryogels to apply a demyelinating agent to slices of mouse brain or spinal cord tissue. Similarly to global demyelination models, the focal application results in demyelination, microglial invasion and remyelination. However, this method results in focal lesions surrounded by healthy tissue, replicating how MS presents in patients. Both grey matter and white matter can be used in this model by using brain slices or spinal cord slices. Up to six brain slices and ten spinal cord slices can be isolated from each animal reducing the number of animals needed for studies of MS.
Presentations from the winners (3Rs Prize Event)
Our 3Rs Prize event featured presentations from both researchers on their winning papers:
About the 3Rs Prize
Co-funded by GSK, the 3Rs Prize is awarded annually by the NC3Rs for a paper that describes outstanding and original work that has or could have major impacts on the replacement, reduction or refinement of the use of animals in research. The competition is open to any research team in the world and applications are assessed by an expert Panel.
The winning researcher was awarded a £28k grant and a £2k personal award. The highly commended winner receives a £4k grant and a £1k personal award.
About GSK
GSK is a science-led global healthcare company. Its purpose is to help people do more, feel better, live longer. Further information can be found on GSK’s website.
About the NC3Rs
The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) is a UK-based scientific organisation dedicated to helping the research community worldwide to replace, refine and reduce the use of animals in research. Primarily funded by the UK Government, the NC3Rs is also supported by the charitable and private sectors. It collaborates with organisations from across the life sciences sector, nationally and internationally, including universities, industries, other research funders and regulatory authorities. Further information can be found on the NC3Rs website.
