Cerebral organoid model wins 3Rs Prize

Work on human cerebral organoids that could replace the use of mice in many aspects of neurological research has won the 2020 3Rs Prize, awarded by the NC3Rs and co-funded by GSK.
Section of a choroid plexus organoid, stained for epithelial cells (green) and stromal cells (magenta).The research represents a major scientific breakthrough because of the capability of these organoids to produce cerebrospinal-like fluid in vitro.
You can view a recording of the award ceremony (conducted over Zoom) on our YouTube channel, or at the end of this news item.
The winning paper from Dr Laura Pellegrini and colleagues at the MRC Laboratory of Molecular Biology describes a method for developing cerebral organoids representative of the choroid plexus, the protective barrier between the blood and the cerebrospinal fluid (CSF), similar to the blood-brain barrier. The paper, published in Science in July 2020, describes a lot of firsts in in vitro neurological research, from accurately modelling the choroid plexus barrier function to their ability to produce and store cerebrospinal-like fluid.
Studying the choroid plexus is particularly challenging as the region lies deep in the brain, preventing patient biopsies from being used in research. The choroid plexus has previously been modelled in vitro using organoid technology, but key features were missing including the barrier function and CSF production which prevented their uptake to replace in vivo studies.
Animal models, predominantly rodents but sometimes macaques, are used in preclinical research to establish the ability of drugs to cross the choroid plexus and into the central nervous system. However, many of these drugs fail in clinical trials, either due to lack of efficacy or an inability to cross into the human central nervous system. Introducing the human organoids described in the winning paper as an in vitro screening platform could help to prevent ineffective compounds progressing to in vivo studies.
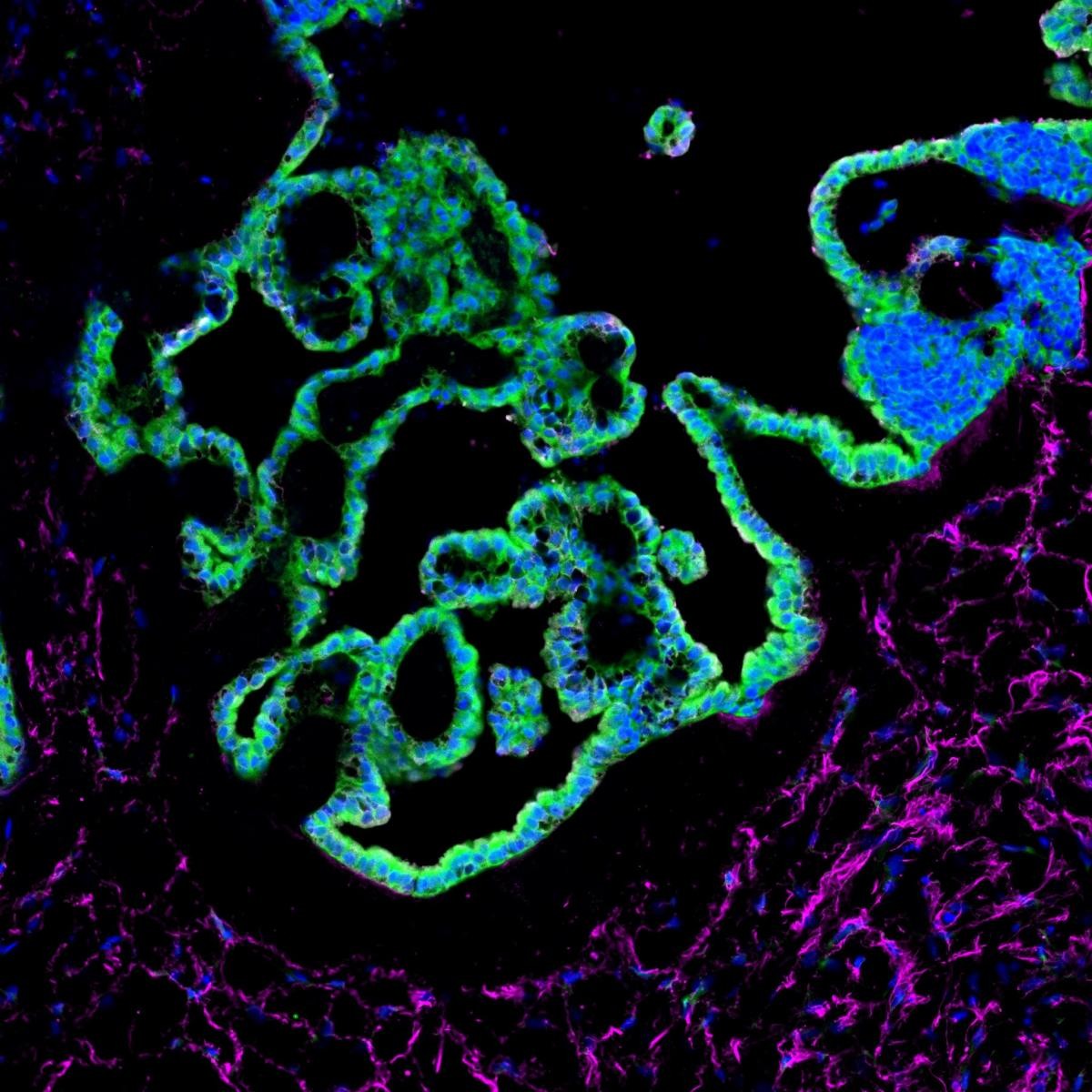
The choroid plexus organoids can also be used to study the ability of pathogens to cross into the central nervous system, including the SARS-CoV-2 (COVID-19) virus. In another publication in Cell Stem Cell, Laura demonstrated how the virus can infect and replicate in choroid plexus epithelial cells, resulting in leakage of CSF proteins. This could explain some of the neurological symptoms seen in COVID-19 patients.
The CSF-like fluid produced by the choroid plexus organoids is stored in small sacs within the organoids and contains biomarkers and proteins similar to human CSF. Developmental diseases such as hydrocephalus or choroid plexus cancers are typically studied using CSF derived from mouse embryos. Approximately 5uL can be retrieved from each embryo, whereas up to 20 times this amount (100uL) can be isolated from one choroid plexus organoid. As they are produced in batches, the process can yield a few ml each time providing a much easier source of CSF which avoids the use of hundreds of mouse embryos.
Laura further describes the development of the choroid plexus organoids and their potential applications in a blog post on The Node. The Prize award will specifically help to investigate the utility of the organoid model to investigate the early molecular mechanisms involved in the removal and clearance of toxic amyloid-beta peptides from the brain. With amyloid-beta pathology key to understanding the progression of Alzheimer's disease, this could lead to the replacement of some of the currently used transgenic mouse models.
In addition, Dr Jennifer Ashworth at the University of Nottingham was highly commended for her work which replaces Matrigel with non-animal-derived hydrogels. Matrigel is derived from mouse tumours and used in many in vitro studies to support cell growth. It is increasingly used in 3D cultures for lab-based models of disease, and approximately one hundred mice can be required to produce the amount of Matrigel needed by a single research institute every year. However, its chemical components are not well defined, affecting the reproducibility of studies
The hydrogels described in the highly commended paper, published in Matrix Biology, can be ‘tuned’ by adding proteins and sugars, altering their stiffness and composition to replicate different in vivo environments. Jenny has used these hydrogels to support breast cancer cells, including cell lines and patient-derived samples, which would otherwise have needed to be sustained using large quantities of Matrigel, or by implanting into mice.
Professor Kevin Shakesheff, Chair of the NC3Rs Board and 3Rs Prize Panel, said: “As ever, the applications received by the Panel were of a very high standard. Both the winning and highly commended publications demonstrate how 3Rs methods can be used to pioneer better science that is more relevant to human health. I am delighted to see how organoid technology has progressed since it was first recognised by the 3Rs Prize in 2013 and look forward to seeing how these two early career researchers will further develop their work in future projects.”
Dr Nick McMahon, VP Non-Clinical Safety, GSK, said: “GSK is delighted to have partnered with the National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs) for the 16th year running to showcase and recognise outstanding scientists positively impacting the 3Rs globally through their ground-breaking research. It is more important than ever to accelerate opportunities where replacement is possible and to embed robust science and welfare practices that reduce and refine the use of animals. GSK's commitment to this prize is a reflection of how our ambition of replacing, reducing and refining the use of animals is integral to our purpose of discovering and developing transformational medicines and vaccines. We hope that these awards will allow our winners to further their exceptional science and inspire many other scientists to pursue the 3Rs within scientific research.”
Winning paper:
Pellegrini L et al. (2020). Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369(6500): eaaz5626. doi.org/10.1126/science.aaz5626.
Highly commended:
Ashworth J et al. (2020). Peptide gels of fully-defined composition and mechanics for probing cell-cell and cell-matrix interactions in vitro. Matrix Biology 85-86: 15-33. doi.org/10.1016/j.matbio.2019.06.009.
About the 3Rs Prize
Co-funded by GSK, the 3Rs Prize is awarded annually by the NC3Rs to recognise work published in the previous three years that has a major impact on the replacement, reduction and refinement of the use of animals in research. The competition is open to any research team in the world and applications are assessed by an expert Panel.
The winning researcher was awarded a £30k grant and a £2k personal award. The highly commended winner receives a £4k grant and a £1k personal award.
About GSK
GSK is one of the world's leading research-based pharmaceutical and healthcare companies. It is committed to improving the quality of human life by enabling people to do more, feel better and live longer. Further information can be found at www.gsk.com.
About the NC3Rs
The UK’s National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) is a scientific organisation dedicated to replacing, refining and reducing the use of animals in research and testing (the 3Rs). Primarily funded by the Government, the NC3Rs is also supported by the charitable and private sectors. It works with scientists in universities and industry in the UK and internationally. Further information can be found at www.nc3rs.org.uk.
