Focus on improving the 3Rs in ocular research

The aim was to improve animal welfare and to make sure that the tests carried out were more accurate.
Allen Pearson, from Origin Product Design, tells the NC3Rs more about the carefully designed plastic device that promises to improve ocular research.
As the population ages, the proportion of people affected by degenerative eye diseases, such as age-related macular degeneration, increases. The treatment of these conditions often involves the use of injections into the eye. Much of the medical research in this area is currently being carried out on rabbits, both in vivo and ex vivo, as their eyes have similar physical properties to human eyes.
The collaborators working on the RETINAS project have developed a device to help scientists improve the process of injecting potential new drugs into rabbit eyes during research. Accurately locating the injection site is difficult since the intended area is only approximately 1 mm in length and is difficult to locate by looking at the external features.
Striving for increased accuracy is highly important, since accidentally nicking the lens whilst injecting can result in potentially serious side effects, ranging from mild inflammation to a significant inflammatory response resulting in early euthanasia of the rabbit or exclusion of data. Improving drug delivery and avoiding the early removal of animals from studies would also reduce the number of animals needed per experiment.
Working together with our industry sponsor allowed Origin to gain a greater understanding of the challenge currently faced by clinicians and scientists during the experiments. The collaboration enabled by CRACK IT gave us the chance to look at the problem from a new perspective and to approach it with the user and the animal in mind.
There were four main areas to consider, if both science and welfare were to be improved:
- Injection site accuracy
- Injection angle
- Injection depth
- Dose accuracy
After a range of initial concepts were developed, a number of 3D printed prototype devices were created. A model eye was also produced from transparent silicone, which was used to test the 3D prototypes. The model eye allowed us to gain a better understanding of the puncture site accuracy and consistency, without the need for animal trials. Use of the 3D printed prototypes gave the researchers an opportunity to experience the benefits, and also evaluate the difficulties, associated with using a device for a task that was, until now, carried out by estimation alone. It showed how difficult the balancing act of placing the device on the eye and then inserting the needle could be, and so highlighted the need for the device to give additional support when in place.

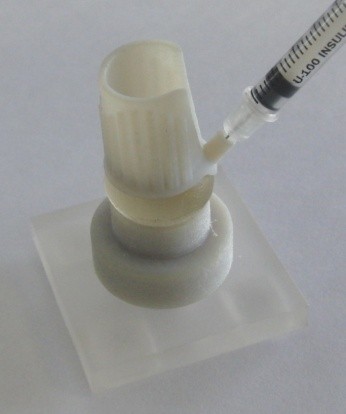
Figures: The 3D printed model eye helped to carry out prototype testing at an early stage
As the material used for the 3D printed prototypes wasn’t representative of the final device, Finite Element Analysis (FEA) simulations were carried out to understand how the device would flex when being placed in the eye and how the plastic would stand up to this. FEA allows for the extremes of performance to be understood without the need to physically replicate them. This was important for the RETINAS project, as it allowed the design process to move forward quickly, crucially without the need for more animal samples.
Once refinements and improvements had been made to the prototype models, the first ex vivo trials were carried out. These trials were the first opportunity to look at the interaction between the device and the rabbit eye itself, and they revealed some unexpected benefits. Amongst other things, not only did the device help to locate the site for insertion, it also helped to reduce the force required for insertion. It was shown to provide a consistent insertion site with no signs of tearing or damage to the outer surface. The testing also highlighted the need for further refinements. For example, the location within the pars plana was found to be a little close to the extremes of the region, so an adjustment was made to bring it closer to the centre of the region.
Once the design had been refined once more, the first set of injection moulded parts was commissioned. A polycarbonate material called Makrolon 2458 was used to produce transparent components. The transparent nature of the device enhances the researchers view of the insertion site, improving the usability even further. The material choice also ensures the device can be sterilised in the test environment, allowing the device to be used for multiple in vivo samples.
A full trial for the device is being carried out by the sponsor and will help to provide statistical data to support its effectiveness. Feedback from both of the collaborating parties already indicates that the device has shown how a user-focused design approach can provide real benefits at reducing the potential for error during testing. Being part of the CRACK IT projects allowed these two different disciplines to be brought together, which may never have happened without it.
