Future Vision: Stem cells and tissue engineering

We caught up with one of the speakers at our Ten Year Vision launch event, Professor Kevin Shakesheff, Director of the UK Regenerative Medicine Platform Hub for Acellular Technologies, who gives us an insight into his hopes for the future of stem cell research.
In 2014 Professor Shakesheff was selected by the EPSRC as one of their ten inspirational leaders in science and technology. He is also a registered Pharmacist and a Fellow of the Royal Pharmaceutical Society and the Society of Biology.
What are the most exciting scientific/technological developments emerging in stem cells and tissue engineering?
It is difficult to know where to start as there is so much going on worldwide in both the fundamental science and clinical translation areas. I’m certain to miss out many important things, but here are a few developments.
The reprogramming of adult cells to form induced pluripotent stem cells continues to transform our thinking about cell therapies. The technology promises to allow adult cells, matched to the patient, to be turned into a cell that can form any tissue in the body. Reprogramming is also being used to turn one type of adult cell into another type without forming a pluripotent intermediate. For example, this could be used to change fibroblasts in the scar tissue of the heart after a heart attack into functional and beating cardiomyocytes.
The formation of a functional thymus is another example of exceptional progress in the field. The use of 3D printing to form scaffolds and tissues is attracting a lot of media attention because it could transform replacement surgery for skeletal tissues. At the resolution of a single cell there are printing or patterning technologies that can achieve the positional accuracy observed within human tissues. The cells that make up a tissue can be printed into position reasonably well. That’s not to say that we can print tissues because we require the cell-to-matrix and cell-to-cell interactions on the nanometer scale to self assemble to complete the tissue structure but that would appear to me to be a problem that can be solved in the future.
Will any of these developments impact animal use?
There could and should be many ways in which stem cell technologies and tissue engineering can impact on the development of replacement technologies. But turning this potential into widely employed techniques will be difficult. There’s a strong momentum helping the clinical application of stem cells. This is driven by obvious unmet clinical needs across many fields of medicine. So the commercial and clinical motivations to solve medical problems with stem cells are easy to define. For replacement technologies we need to define the problems that could to be solved by stem cells technologies. For a researcher, such as me, who focuses on clinical applications but would like to contribute to replacement technologies, it can be difficult to find a way into the field. The CRACK IT initiative is a good example of defining problems that will stimulate multidisciplinary research.
What are the biggest challenges in research involving animals in your field?
Regenerative medicine therapies, using stem cells or tissue engineering concepts, are difficult to test for safety and efficacy. There are numerous reasons for this difficulty. One prime reason is the length of time it takes a cell therapy to regenerate a tissue within the body. Cartilage, bone, cardiac muscle and most other tissue targets regenerate over periods of many months. These timescales requires animal models to be run for long periods otherwise efficacy will not be evident. The size of tissues and rate of repair are very different in rodent models compared to humans, so we have to be careful when interpreting small animal data in preclinical studies.
Where would you like to see the field of stem cells and tissue engineering in 10 year’s time?
My first wish would be for at least one major clinical breakthrough in an area that is untreatable by current medicine. Functional improvements in patients after a stroke or spinal cord injury, the reversal of cardiac muscle damage or long term function of islet cells in diabetic patients are all examples that could transform medicine and appear realistic, if difficult, targets for the next decade. Next I would like to see commercial successes that stimulate an increase in investment and help build an industry for the future in the UK.
What would be the single biggest change needed to achieve this?
We need multidisciplinary teams to consider the development of a stem cell based medicine as a whole. Stem cell therapies have the best, and possibly only, chance of success if we consider every aspect of their function from the earliest stages of manufacture through regulatory approval and finally to how they function in the patient. The UK is embracing this multidisciplinary approach with the Cell Therapy Catapult, EPSRC Centre for Innovative Manufacturing, the five UK Regenerative Medicine Platform Hubs and other national initiatives.
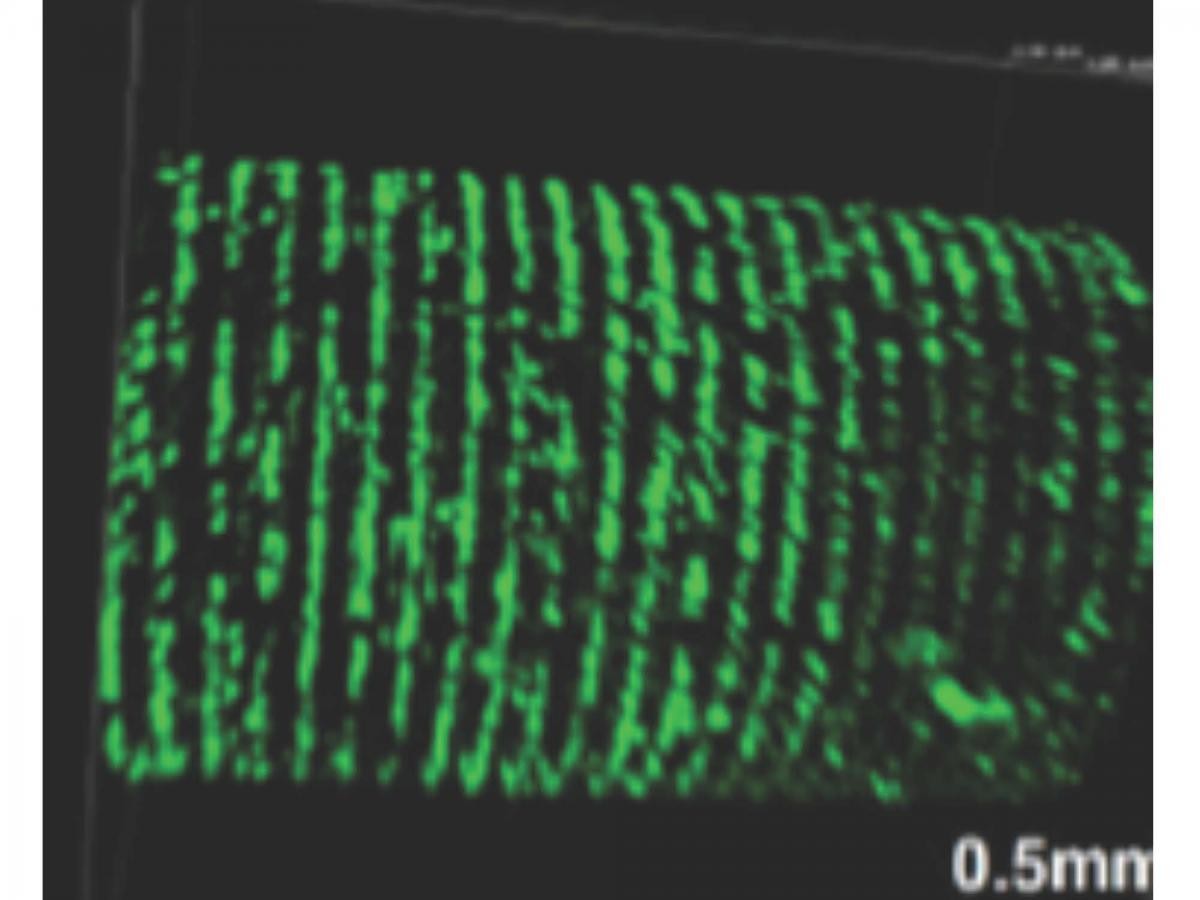
Patterning of human cells to form tissue-like constructs
