New international project to improve environmental toxicology testing in fish and reduce animal use

OECD to develop new guidance to minimise fish use in some studies, based on NC3Rs-led work.
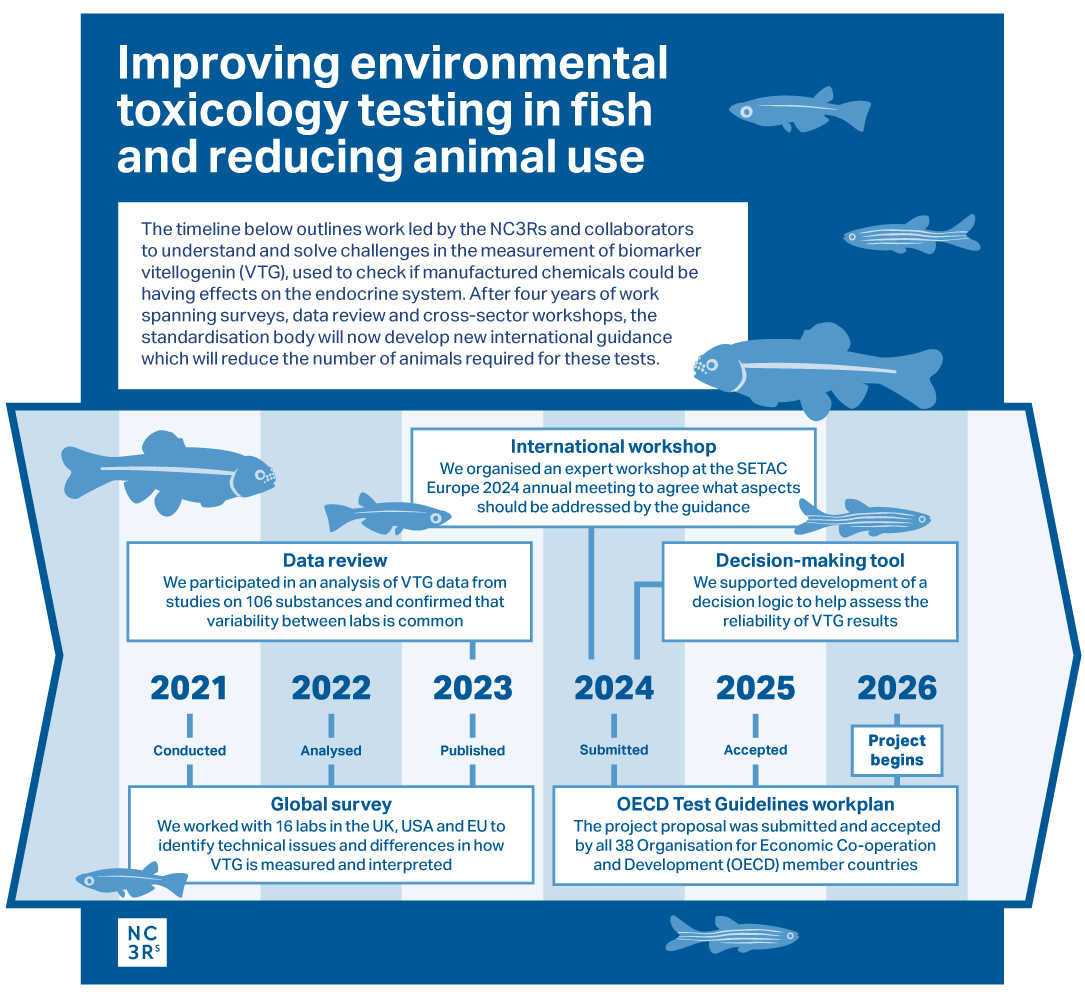
The Organisation for Economic Co-operation and Development (OECD) – the body responsible for setting international standards in chemical safety testing – has agreed to develop new guidance on measuring the biomarker vitellogenin (VTG) in fish. VTG assessment is carried out in zebrafish, Japanese medaka or Fathead minnow to check if chemicals in the environment could have effects on the endocrine system and these studies are important for protecting wildlife populations. Concentrations of VTG mRNA or protein are assessed in blood plasma, liver, head and tail homogenate, and whole-body homogenates. The data obtained can be highly variable within and between labs (even for control fish) and this can mean animals may be wasted or additional studies triggered to address uncertainties in the findings. VTG is assessed in multiple tests with some studies requiring around 3,000 fish.
The new guidance will provide clear best practices for laboratory measurements and statistical analysis to make results more reliable, reduce repeated or additional testing and ensure that laboratories around the world use the same approach. This is a result of several years of NC3Rs-led and collaborative work to understand and solve challenges in VTG measurement which impact on fish use, with input from contract research organisations, companies and regulators who conduct, submit and assess the tests.
Dr Natalie Burden (NC3Rs Head of NAMs Strategy) will be the UK’s project lead for the delivery of the new guidance which will be developed in collaboration with an expert OECD working group. This builds on Natalie’s expertise in developing the evidence base to improve VTG assessment, generating consensus across international stakeholders and sectors and gaining the support of OECD member countries to develop the guidance. Natalie recently led an expert group, with regulatory agencies and industry, to evaluate the impact on animal use of proposed endocrine disruptor assessment requirements, including studies where VTG would be assessed, under EU REACH. The evaluation highlights that between 40,000 to 69,000 studies could be required overall at a cost of between €17 to €24 billion and requiring between 14.30 and 25.77 million animals.
Development of the VTG guidance document will officially begin in early 2026. Once complete, it will become the go-to international resource for labs, industry and regulators involved in VTG assessment, improving data quality and interpretation and ultimately reducing the number of fish used in endocrine disruptor testing.
Read the workshop report that led to the OECD proposal, co-authored by 42 experts from regulators, industry, academia and method developers from around the world: Scoping for the development of a proposal for an OECD guidance document on fish vitellogenin assessment.
Learn more about our previous work and publications in this area: Identifying and addressing challenges in fish vitellogenin assessment.
Learn more about analysis led by the NC3Rs to inform new endocrine disruption testing requirements in the EU to avoid substantially increasing animal use and resource.

