Single use of needles: putting refinement into practice
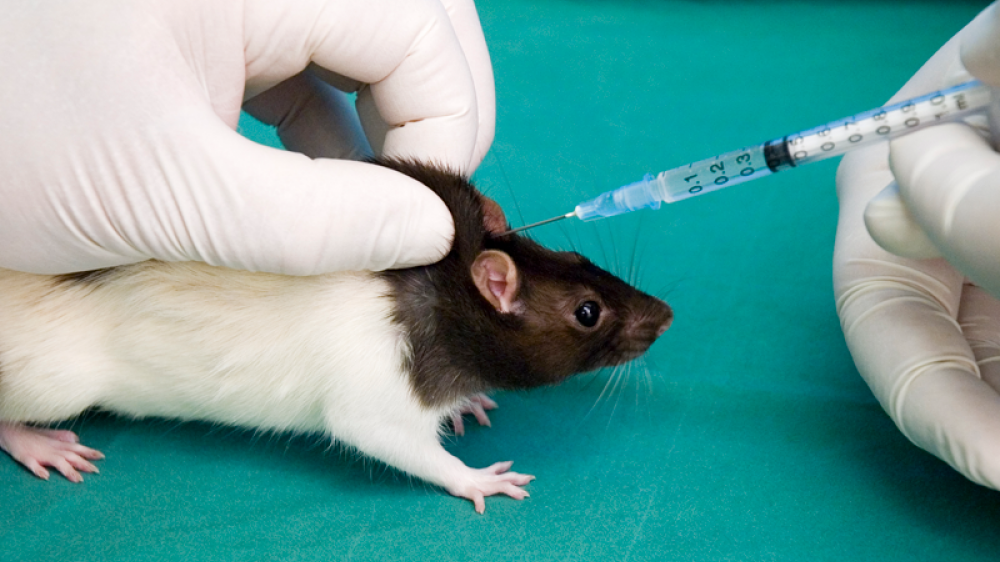
In this guest blog post, Dr Sally Robinson, Director of Animal Sciences and Technologies at AstraZeneca (Alderley Park), explores how the company has implemented the single use of needles as a refinement across their sites.
Since the NC3Rs' first blog post on needle re-use, there has been a lot of interest and discussion across establishments about the evidence base to support the single use of needles and in the UK the Home Office have adopted this as a themed inspection area in 2019.
Working in large organisations can make dissemination of good practice in connection with animal welfare difficult. Many pharmaceutical companies have the added complication of operating across a range of cultures and legislative frameworks. At AstraZeneca the Council for Science and Animal Welfare (C-SAW) is the expert group leading our global approach to animals in research, promoting the application of the 3Rs, supporting openness about animal use, and providing assurance that we meet our standards. C-SAW membership includes veterinary, laboratory animal scientist, statistician, research scientist and compliance roles.
Local ethical oversight bodies (e.g. AWERB in the UK, Djurskyddsorgan in Sweden, IACUC in USA) have an important role to support implementation of refinement and so we have focussed on how to provide support to staff using animals by providing advice to oversight bodies through global good practice statements. The statements are formulated from available evidence and literature and to reflect the professional consensus opinion of C-SAW members and are designed to provide consistent advice to the oversight bodies in developing their local policies and procedures.
Our first Statement of Good Practice was on the topic of the administration of substances to animals by parenteral routes and one of the critical aspects covered was needle use.
The use of an individual needle for each animal is a good example of where the evidence base to support a change in practice is complicated. Since the first blog post many people have started to look closely at their needles - some have reported that the needles don’t dull in their hands while others have seen that they do. We may never be 100 % certain that every time a needle is reused it will become dull as factors around injection such as needle brand, size, and type of needle, species of animal, training and expertise of the operator, nature of the substance are as important as the number of times a needle gets used. However, there is always a risk that the needle will be dull after the first use and in the absence of certainty the precautionary principle means we should give the animal the benefit of the doubt. This is the approach we have taken at AstraZeneca in our Statement of Good Practice. Through our collaborative discussions across sites and research areas the following high level principles were agreed.
- The fundamental principle is every animal deserves a clean sharp needle. There is a risk of both cross contamination and dulling of the needle through re-use. That is not to say the risk will always be realised but the risk is there.
- The best way to achieve the fundamental principle described in 1 above is to use one needle per animal. This is good practice given one of the underlying principles of the 3Rs is that procedures applied to animals must be refined to reduce to the minimum any possible pain, suffering, distress or lasting harm.
- Single needle use is described as the default position within the Good Practice Statement. The oversight bodies across sites can provide mechanisms to review any rationale for any deviations from the default position.
One of the reasons for re-use of needles and syringes is loss of valuable material (such as cells, compounds, viruses) due to dead space in syringes and needles. This is most relevant in mice given their size and the small volumes administered mean dead space losses can be a significant percentage of the material.
Within AstraZeneca one of our key therapy areas is oncology. For the animal models it is usually necessary to inject living cancer cells into mice so that tumour development and the effects of treatment can be assessed. The increased excess required to cover the dead space losses through single needle use can put harvesting quality cells at risk for certain cell lines. Compromising the cell quality may have a negative impact on tumour development thus putting the study at risk of failure. In these cases, a local harm/benefit discussion will balance the risk of re-using a needle versus the risk of compromising cell growth within the mice. Where there is scientific rationale for exceptions, needle reuse is confined to a cage of mice to minimise potential for cross-contamination and if there is evidence a needle is becoming dull before that point it is changed.
We have found there is considerable value in this collaborative approach to outlining good practice.
- The company view can be outlined and disseminated in a consistent manner across global sites. This provides a mechanism for individual sites to review and assess their local practice and identify any gaps.
- Local oversight bodies bring together a range of perspectives and serve as good forum for conducting harm/benefit discussions.
- Having the discussions with scientists as the good practice statements are developed allows for a more collaborative approach to refinement.
