Using fewer animals to assess environmental safety

A recent cross-sector review has highlighted several key approaches available for ecotoxicologists and risk assessors to evaluate potentially hazardous chemicals, while minimising the use of vertebrates.
The Focus article has been published in Environmental Toxicology and Chemistry by the steering committee of the Society of Environmental Toxicology and Chemistry (SETAC) Animal Alternatives in Environmental Science Interest Group.
SETAC is the major society connecting environmental scientists, with over 6,000 members from almost 100 countries. The role of the Animal Alternatives Interest Group is to foster discussion of key technical challenges for the future of the 3Rs in environmental science. The team who put together this publication includes contributors from regulatory bodies, academia, industry and SMEs from Europe and North America. Dr Natalie Burden, one of our Programme Managers for toxicology and regulatory sciences, is a co-author of the paper.
The review describes the issues around vertebrate ecotoxicity testing that result from the need for companies to generate in vivo data to assure regulatory authorities that products are safe. This includes discussion on the opportunities for approaches that replace, reduce or refine the use of vertebrate animals within current regulatory frameworks. One option highlighted is the use of computational methods to assess the safety of pesticides, and their metabolites in particular. We have an ongoing project in collaboration with three agrochemical companies to provide the scientific evidence base to support the use of such models in place of fish acute toxicity tests for this purpose.
The article also refers to the available non-animal methods for the testing of fish bioaccumulation. Information from animal bioaccumulation studies is currently required before marketing plant protection products and industrial chemicals under various global regulations, which results in the use of an extensive number of fish. We continue to lead a project exploring the option to reduce the number of substance concentrations tested in these studies, which would ultimately decrease the numbers of fish used by one third.
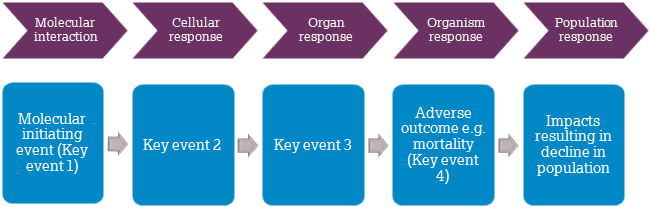
The value of the adverse outcome pathway (AOP) framework is also highlighted as a long-term prospect to reduce reliance on animal toxicity testing. The vision is that non-animal approaches could be used to determine whether a chemical or drug of interest causes the critical (or ‘key’) events within the biochemical pathways, or AOPs, known to result in adverse outcomes in organisms or populations (see image). Advances in this area could ultimately enable the prediction of an adverse outcome within an organism in the absence of animal studies. We are working with the scientific community through a large-scale programme to advance approaches that use the AOP framework for both human and environmental safety assessment purposes.
Finally, the varying regulatory data requirements between geographical regions are highlighted as a critical issue leading to surplus vertebrate testing, as companies often seek to market their products globally. We are working on a number of new initiatives to address this, as better harmonisation and mutual acceptance of data from studies across different regions could lead to a substantial reduction in the number of animals used in both regulatory ecotoxicity and human toxicity testing.
For more information, visit our 3Rs in toxicology and regulatory sciences resource hub or sign up to our quarterly newsletter (‘Tox News’).
