CRACK IT Challenge
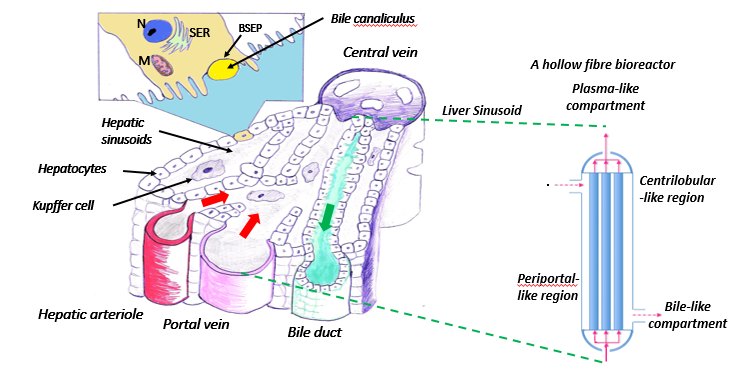
IVIVE: Development and mathematical modelling of zonated hollow fibre bioreactors for in vitro to in vivo extrapolation of systemic chemical toxicity
At a glance
Completed
Award date
December 2011 - September 2016
Contract amount
£997,634
Contractor(s)
Sponsor(s)
R
- Replacement