Replacing mice and improving Glioblastoma outcomes
Professor Anthony Chalmers and Co-Investigator Dr Natividad Gomez-Roman have further developed a 3D human cell culture model replacing the use of mice to evaluate and screen treatments for Glioblastoma (GBM).
Representing human cancer biology
GBM is the most common type of brain cancer but patient outcomes remain poor. Tumours are inherently resistant to both chemotherapy and radiotherapy, complicating treatment options. A number of potential new drugs have failed during late-stage clinical trials, suggesting the widely used preclinical screening method, the mouse xenograft model, does not accurately reflect key aspects of human disease. These experiments involve transplanting cancer cells into immunocompromised mice and monitoring tumour growth. Orthotopic xenografts, where GBM cells are implanted into the mouse brain, are more relevant to the clinical situation than subcutaneous xenografts but are technically challenging.
In vitro alternatives were limited to 2D GBM cultures, which had also failed to improve clinical outcomes. Anthony was awarded an NC3Rs Project grant to further develop a 3D GBM cell culture system and demonstrate its utility for testing new therapeutics. Crucially, the system includes GBM stem-like cells (GSCs), known to contribute to treatment resistance, alongside human brain endothelial cells. This creates a multicellular model of the ‘perivascular niche’ – the microenvironment around blood vessels in the brain in which GBM tumours thrive – which more closely reflects human cancer biology.
From cells to screening tool
Anthony’s NC3Rs Project grant supported him to apply the 3D GBM cell culture model to identify and evaluate new molecular targets for GBM therapies. In particular, he has used the model for preclinical screening of radiotherapy sensitising drugs. The in vitro GBM model not only replaces the widely use mouse xenograft model, but also improves the accuracy of preclinical drug evaluation. The system can replicate radiotherapy-drug combination clinical trial data to within 5% accuracy, allowing for faster screening for effective treatments in a more human-relevant model.
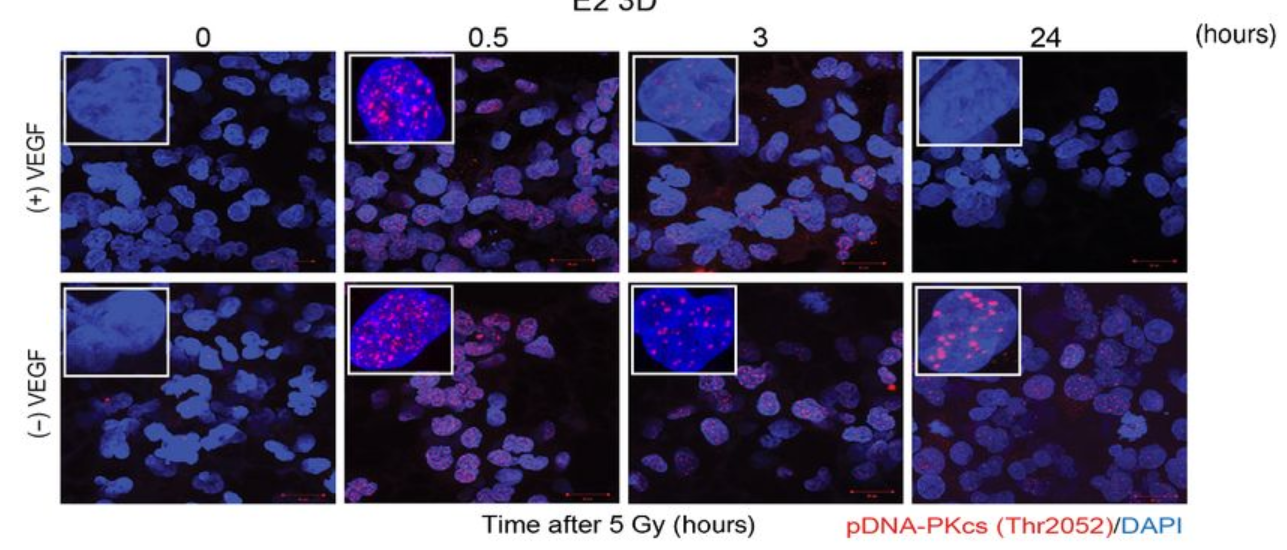
The model has attracted pharmaceutical and biotechnology companies to fund its use to evaluate and screen potential GBM treatments. As part of an ongoing collaboration with BenevolentAI, Anthony’s lab screened approximately 100 radiotherapy sensitising drug combinations selected by AI-enabled target identification. Through an extensive network of collaborations and dissemination through publications (in Nature, Molecular Cancer Therapeutics and Cancers), Anthony’s 3D cell culture model is now used by scientists across the country and internationally, both in academia and industry, to accelerate the development of novel GBM therapies.
Building on the success of the NC3Rs Project grant, Anthony has secured £4.4M in research grants to continue developing the in vitro system and increase its utility to replace mice in the preclinical assessment of GBM treatments. Anthony and Natividad were also awarded an NC3Rs Technologies to Tools grant, in collaboration with Milner Therapeutics Institute at the University of Cambridge, to investigate the genetic changes in GBM cells which lead to radiotherapy resistance and develop the model into a high-throughput tool to increase GBM drug development efficiency.
Natividad is now head of her own research group, at the University of Strathclyde, where the work she did on the GBM model contributed to receiving her Chancellor’s Fellowship at the Strathclyde Institute of Pharmacy and Biomedical Sciences. She has also secured £420k in further funding to both apply and further develop the 3D cell culture system to investigate other brain and ovarian cancers.
Embedding the 3Rs in cancer research and clinical culture
Anthony co-leads a radiotherapy drug combination working group of nine universities, research institutes and hospitals that engage with industry on collaborative projects to put replacement methods into practice in cancer research. Alongside driving the adoption of his replacement approach, Anthony is working to raise awareness of the 3Rs and their scientific benefits beyond the research community. He has developed and delivered radiobiology training for all oncology trainees in Scotland to embed the 3Rs in the clinical culture and presented his 3D model to an all-party parliamentary group on brain tumours in 2022.
Hear more from Anthony and Natividad on the impact of the NC3Rs Project grant:

