Screening chemoprevention strategies for squamous lung cancer
Dr Frank McCaughan and student, Dr Phil Barry, miniaturised a human cell-based model of early-stage lung cancer enabling the assay to replace some drug screening experiments using mice.
Bronchial dysplasia – a precursor to lung cancer
Lung cancer has one of the highest death rates of all cancers, partly due to patients presenting at a late stage when the disease is incurable. Developing chemoprevention strategies for high-risk patients and improving early detection of lung cancer are key focuses of the scientific community. Research is ongoing to identify molecular events in developing malignancies that can be used as drug targets.
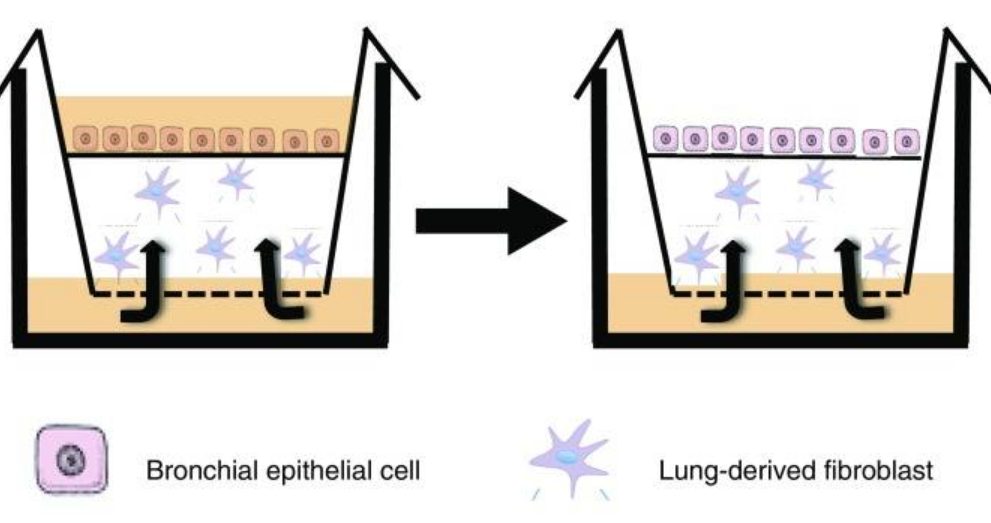
Cells undergo a series of changes before becoming cancerous. Initially cells enter a state of hyperplasia, where there is an increase in cell numbers, followed by dysplasia, where cells display abnormal characteristics. While these changes always proceed cancer developing, not all hyperplastic or dysplastic cells will progress to becoming cancerous. To better understand the main events that define progression from dysplasia to squamous lung cancer, Dr Frank McCaughan and colleagues developed a 3D in vitro cellular model of bronchial dysplasia.
Moving from mechanisms to therapeutic targets
The model Frank and colleagues developed uses immortalised human bronchial epithelial cells. These are grown at the air-liquid interface, with the base of the cells in contact with culture media and the cell surface exposed to air, similarly to cells in the lung. Using this model, Frank’s lab identified that dysregulation of a gene called SOX2 was sufficient to drive cells to a dysplastic phenotype and had the potential to be used as a therapeutic target.
Frank was awarded an NC3Rs Studentship to further develop the in vitro assay and enable potential chemoprevention therapeutics to be screened, replacing the use of mice in these studies. Phil, the PhD student, was able to miniaturise the assay making it higher throughput for screening purposes. To continue to unpick the mechanisms of cancer development, build confidence in the model and demonstrate its utility, Phil analysed downstream targets of deregulated SOX2. By blocking specific signalling pathways, Phil was able to determine which proteins are necessary for maintaining the dysplastic phenotype and identify a potential therapeutic vulnerability that could be targeted with treatments. Frank estimates that his laboratory replaced over 1,500 mice using the assay to identify these targets rather than performing in vivo studies.
Replacing mouse use in industry
Frank established new collaborations with Janssen and AstraZeneca based on the data from Phil’s PhD and received further funding to screen therapeutics to reverse bronchial dysplasia using the miniaturised assay. In a typical in vivo screening experiment, multiple doses of each drug are tested using mice that have been bred to possess specific mutations reflective of squamous lung cancer. For every mouse needed in an experiment, approximately 15 mice are needed in the breeding programme. Depending on the study, either naphthalene, an organic compound toxic to the airway epithelium, is administered to animals via inhalation with cancer induction occurring within six months or for carcinogen-based models NTCU (an organic compound) is painted on the skin repeatedly for six months.
Looking to the future – a bioengineered model of the lung
Epithelial cells line the respiratory tract, from the nose through to trachea to the bronchi and bronchioles. The entire surface exists as one layer, but the cellular composition varies along the tract. Currently, complex in vitro models, such as the one developed in this Studentship, replicate one part of the epithelial layer as a discrete unit and do not include the full integrated epithelium. Frank and colleagues have been awarded over £1M in funding from BBSRC, expanding on the success of the bronchial epithelial model to create an in vitro “lung”. Each “lung” will be developed using multiple cell types from an individual patient to enable studies into lung biology, the impact of the different cell types and analysis of personalised treatments for patients.
We have an entirely new programme building lungs in the laboratory and this would not have happened without the confidence in in vitro human models engendered by our NC3Rs funding.
Dr Frank McCaughan

