CRACK IT Solution
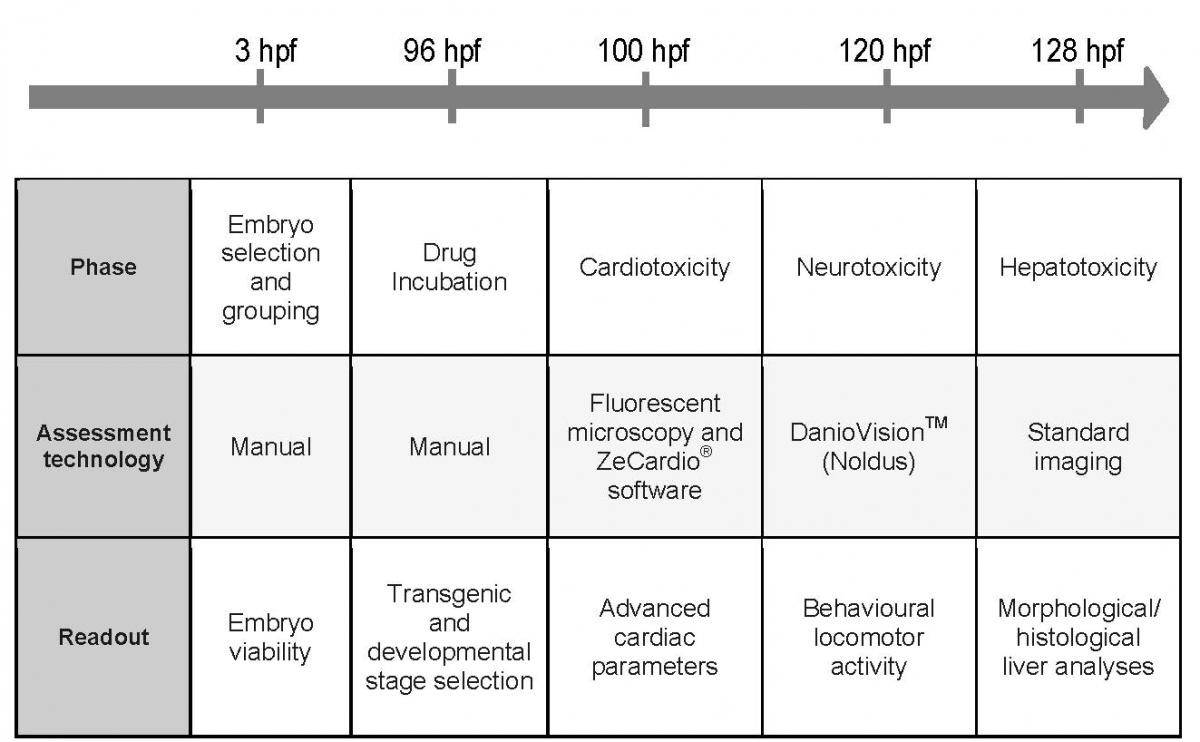
ZeGlobalTox: multi-organ toxicity testing in zebrafish

At a glance
Completed
Award date
June 2015 - June 2016
Contract amount
£30,000
Contractor(s)
R
- Replacement