Review of the use of two species in regulatory toxicology studies
At a glance

Overview
Current regulatory guidelines usually require safety and tolerability data from two species, a rodent (rat or mouse) and a non-rodent (dog, minipig or non-human primate), before administration of potential new medicines to humans in the first clinical trials. In the UK in 2020, animals used for repeat dose toxicity testing purposes (just one of the safety tests conducted) amounted to 10,670 experimental procedures using mice, 27,432 experimental procedures using rats, 2,082 experimental procedures using dogs and 1,142 experimental procedures using non-human primates (data from 2020 Home Office statistics).
The UK is only one of the countries performing these tests and it is impossible to estimate how many animals are used for this purpose worldwide. Many of the regulatory toxicology tests used today were introduced 30 or 40 years ago – the pharmaceutical industry has changed considerably in the intervening years with new drug targets, new types of compounds and new in vitro and in silico technologies available to evaluate safety. Where appropriate, regulatory requirements have evolved and been updated to adopt in vitro approaches, refine in vivo tests and reduce the numbers of animals used. This project, funded by the Association of the British Pharmaceutical Industry (ABPI), is a further opportunity to review whether the standard testing paradigm can be modified and provide an evidence base for updates with regards to the need for two species.
The Two Species project
An international expert working group was formed in 2016, consisting of 37 organisations (pharmaceutical/biotechnology companies, contract research organisations, academic institutions and regulatory bodies). We have reviewed how and when two species are used within pharmaceutical regulatory toxicology studies, and whether a rodent and a non-rodent species are still required in the current industry landscape. The initial focus for the project has been on whether existing opportunities to use a single species are being fully exploited and/or could be expanded, without detrimental effect on human safety.
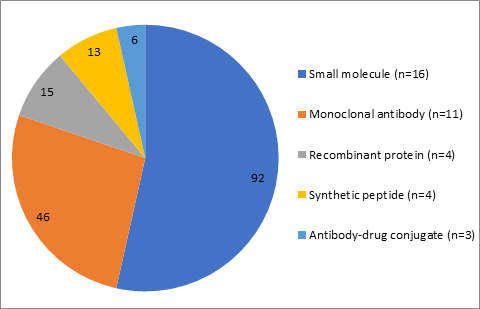
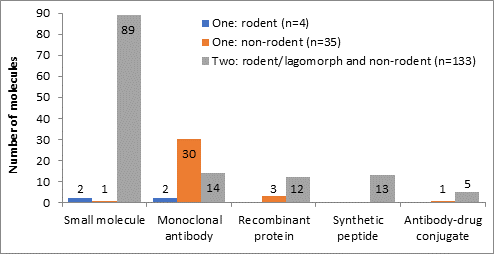
Within the current paradigm for the development of biologicals, reduction to a single species for longer-term studies (the 13, 26 or 39 week toxicity studies to support long-term dosing in humans) may be possible if similar target organ toxicity profiles are identified in two species within the short-term studies (the 4 week toxicity studies to support first administration in humans), as outlined within the ICHS6(R1) guideline. We have collected preliminary evidence that supports this, and for expansion of the opportunities to other modalities (e.g. small molecules). However, applying these opportunities to the current nonclinical toxicology strategies for other molecule types and associated changes in the regulatory guidelines would need more robust evidence to demonstrate minimal risk/impact for human risk assessment.
This work has been published in Regulatory Toxicology and Pharmacology [2] – we have also produced a summary of the findings [3].
Related Content
- News: Opportunities for use of a single species in drug development.
- News: Launch of a new NC3Rs-ABPI collaboration.
- Additional data: Reducing the use of recovery animals.
- News: CRACK IT Challenge 40: Virtual second species.
Two Species Phase II: ICH M3(R2)
In 2023 we launched a new phase of the project to review opportunities to expand the use of a single species in long-term toxicity studies to molecules following the ICH M3(R2) guidelines.
Publications
- Prior H et al, (2022). Exploring the Definition of “Similar Toxicities”: Case Studies Illustrating Industry and Regulatory Interpretation of ICH S6(R1) for Long-Term Toxicity Studies in One or Two Species. International Journal of Toxicology 41(3), 171–181. doi: 10.1177/10915818221081439
- Prior H et al. (2020). Justification for species selection for pharmaceutical toxicity studies. Toxicology Research 9: 758-770. doi: 10.1093/toxres/tfaa081
- Prior H et al. (2020). Opportunities for use of one species for longer-term toxicology testing during drug development: a cross-industry evaluation. Regulatory Toxicology and Pharmacology 113: e104624. doi: 10.1016/j.yrtph.2020.104624
- Summary of findings (Q&A). Opportunities for use of a single species in drug development. 2020.
- Prior H et al. (2019). Integration of Consortia Recommendations for Justification of Animal Use within Current and Future Drug Development Paradigms. International Journal of Toxicology. 38: 319-325. doi: 10.1177/1091581819852922
- Prior H et al. (2018). Reviewing the Utility of Two Species in General Toxicology Related to Drug Development. International Journal of Toxicology 37(2): 121-4. doi: 10.1177/1091581818760564
- Prior H et al. (2020). Incidence of new toxicities in biologics and small molecules upon long-term dosing. The Toxicologist 174(1): #3404. Late-breaking poster presented at SOT, March 2020.
- Prior H and Sewell F (2019). Are We Fully Exploiting the Use of a Single Species for Post-”First-in-Human” (FIH) Studies Allowed within ICHS6? The Toxicologist 168(1): #2117. Poster presented at SOT, March 2019.
- Prior H et al. (2018). Can we expand the use of one species for post-First-in-Human (FIH) studies, within and beyond ICHS6? Poster presented at ACT, November 2018.
- Prior, H et al. (2018). Reviewing the use of two species in toxicology studies supporting drug development. Toxicology Letters 295 (S1): S222-S223. Poster presented at EuroTox, September 2018.
