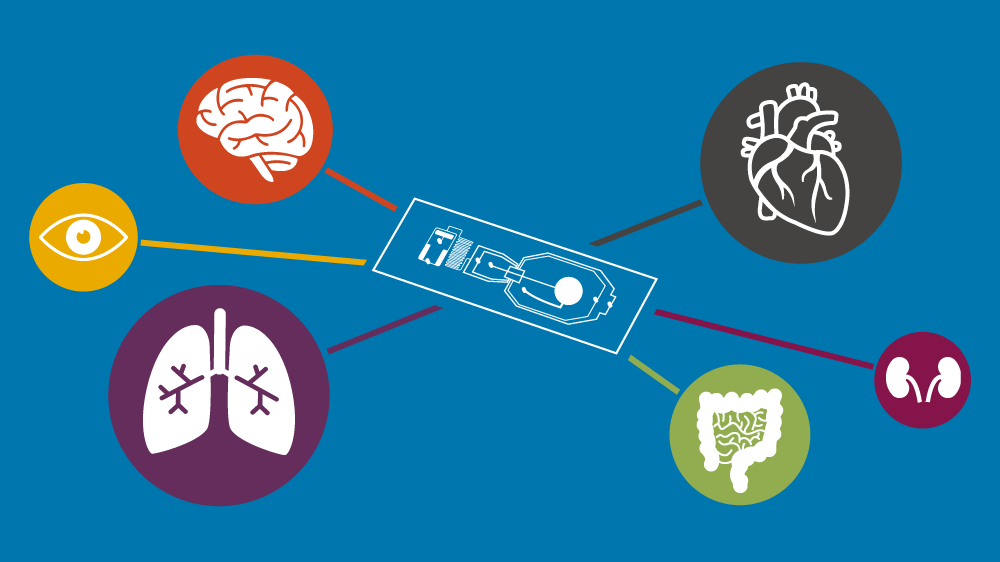
Microphysiological systems
Microphysiological systems (MPS) are experimental platforms which provide an alternative to using animal models in basic and applied research.
On this page
Introduction
Microphysiological systems are in vitro platforms composed of cells or tissues of human or animal origin exposed to a microenvironment designed to mimic the physiological aspects of tissue and organ function. MPS design may aim to support cultured cells with physical (e.g. temperature, pH and oxygen), biochemical, electrical, mechanical (e.g. flow or stretch), structural or morphological conditions to recapitulate a set of specific properties that define a healthy or diseased organ or tissue beyond conventional static cell and tissue cultures. Examples of MPS are organ-on-a-chip systems, organoids, 3D co-cultures with matrix and 3D bioprinted tissues.
The PDF below provides an overview of what MPS are, why they're important to the 3Rs and key developments.
NC3Rs work across MPS
We support the development and uptake of MPS through a variety of mechanisms including:
- Funding research and innovation.
- Collaborating nationally and internationally.
- Disseminating opportunities through our events and webinars.
Below you can find out more about our MPS activities as well as other global programmes and initiatives. The aim of these resources is to showcase our portfolio of projects in this area and provide information and support for the community, including technology developers, end-users, regulators and in vivo researchers who are looking to adopt alternative approaches.
Reports and recommendations
NC3Rs report
This report, published by the NC3Rs in 2021, summarises the current OoC landscape and our recommendations for supporting the technology's development and wider adoption.
Workshop summary report (2018)
A joint report from Medicines Discovery Catapult, the Medical Research Council Centre for Drug Safety Science and the NC3Rs summarising an OoC workshop held in 2018, outlining the key focus areas for the UK to advance the technology.
MPSCoRe working group
We have established with NICEATM (the US National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods) a working group to coordinate global efforts for the use of microphysiological systems (organ-on-a-chip and other complex multicellular in vitro models) to reduce reliance on in vivo studies of COVID-19.
Learn more about the MPSCoRe working group in Our portfolio.
Funding opportunites
We fund the development and application of MPS across our research funding and CRACK IT innovation programmes, and encourage applications in this space. To date (March 2023) we have committed £28.6M funding to research using MPS. This includes developing new MPS models for studying diseases such as cancer, cardiovascular disease and Alzheimer’s disease, facilitating the transfer of MPS between research labs to increase their use, and using MPS to assess kidney CNS toxicities.
We fund new model development through our Project grants and PhD Studentships.
Our CRACK IT innovation programme champions innovation in the 3Rs, turning great ideas into products and services.
If you are an end-user, you may be interested in submitting an idea for a CRACK IT Challenge through our annual open call (e.g. a call for a new product or technology targeted to your needs). Visit the Sponsor a Challenge page on our Innovation Platform website to find out more.
To learn more about the MPS technology we have supported through our research funding and CRACK IT programmes, visit Our portfolio.
Global programmes and initiatives
Funding and partnerships have been critical in supporting MPS development and moving it towards commercialisation and adoption. The list below summarises some of the past and ongoing global programmes and initiatives in the MPS field.
- The BioSystics Analytics Platform
- The SMART Organ-on-Chip consortium
-
The Defense Advanced Research Projects Agency (DARPA) microphysiological systems programme
-
The Innovation and Quality (IQ) consortium MPS affiliate (IQ MPS affiliate)
-
The 3Rs Collaborative (3RsC) Microphysiological Systems Initiative
-
The Netherlands Institute for Human Organ and Disease Model Technologies (hDMT)
-
The Centre for Alternatives to Animal Testing (CAAT)/CAAT Europe T4 Think Tank on MPS
The BioSystics Analytics Platform
Previously the Microphysiological Systems Database, The BioSystics Analytics Platform is a web-based resource which aims to accelerate the development and application of MPS for basic research and drug development. It collates and makes accessible experimental data submitted by MPS model developers and preclinical and clinical data from a range of open source and proprietary databases including PubChem, CheMBL, Genbank, PubMed, ClinicalTrials, OpenFDA and EMA. The database includes functionality to manage, analyse, share, computationally model and integrate data in one platform, as well as tools to assess the reproducibility and transferability of MPS experimental models.
The SMART Organ-on-Chip consortium
Funded by the NWO-TTW Perspective Programme of the Dutch Research Council, The SMART Organ-on-a-Chip consortium is a multidisciplinary programme which is bringing together engineers and biomedical researchers with end-users to develop a novel, Standardised, open and Modular OoC Approach to Recapitulate Tissues (“SMART OoC”) to overcome barriers to adoption of organ-on-a-chip technology.
The Tissue Chip for Drug Screening Programme
Led by the National Centre for Advancing Translational Sciences, part of the National Institute of Health, this programme was launched in 2012 with the aim to develop tissue chips of human organ systems to better predict drug safety and efficacy. A range of platforms have been developed and two Tissue Chip Testing Centres and a Tissue Chip Database Centre were established to independently test and validate these platforms. Focus areas for this initiative included tissue chips for disease modelling and efficacy testing, tissue chips for modelling the immune system, tissue chips for pain, opioid addiction and overdose, clinical trials-on-a-chip, tissue chips in space and tissue chips for studying COVID-19. The Tissue Chip Testing Center project period closed in 2021 and this research now continues through companies and consortiums established by the team.
Papers describing the programme and outputs:
The Defense Advanced Research Projects Agency (DARPA) microphysiological systems programme
The programme was launched in 2012 to develop an in vitro platform linking ten or more organ systems together to evaluate the safety and efficacy of novel medical countermeasures (e.g. emerging infectious diseases and chemical or biological attack). Funding was awarded to teams from the Massachusetts Institute of Technology (MIT) and the Wyss Institute, which led to the development of two platforms. The DARPA report summarises the programme and its outputs.
Papers describing the platforms developed:
The Innovation and Quality (IQ) consortium MPS affiliate (IQ MPS affiliate)
A not-for-profit organisation of pharmaceutical and biotechnology companies to facilitate cross-pharma collaboration and data sharing of MPS, raising awareness of and supporting MPS qualification and implementation.
A series of papers was published in Lab on a Chip outlining the pharmaceutical industry perspectives and considerations for developing, evaluating and characterising MPS models to support drug discovery and development – see Fabre et al. (2020). A follow up set of papers were published in Altex Alternatives to Animal Experimentation in 2023 – see Irrechukwu,et al (2023).
The 3Rs Collaborative (3RsC) Microphysiological Systems Initiative
The NA3RsC established the MPS initiative to encourage collaborations to increase adoption and regulatory acceptance of MPS technology. The initiative includes representatives from providers of commercial systems and end-users. The NA3RsC Microphysiological Systems Technology Hub highlights commercially available MPS models. These include models provided by Alcyomics, Newcells and Mimetas which were developed with NC3Rs CRACK IT funding. Models available on the Hub include organ-on-a-chip, organoids and spheroids and the list is searchable by tissue type.
The German GO-Bio multi-organ bioreactor programme
A GO-Bio grant was awarded to the Technisch Universitat Berlin in collaboration with TissUse GmbH and the Fraunhofer IWS in Dresden and the Fraunhofer IGB in Stuttgart.
Papers describing the multi-organ models developed including a human 3D liver and skin model and an ADME chip connecting human intestine, skin, liver and kidney:
EU body-on-a-chip
Funding was awarded to a consortium including InSphero AG (Switzerland), ETH Zurich (Switzerland)), KU Leuven (Belgium), Technical University Dortmund (Germany) and AstraZeneca from 2012 to 2015 to develop a device that interconnects multi-organ tissue models. This led to the generation of a plate-base device commercialised by InSphero as the AkuraTM Flow.
The Netherlands Institute for Human Organ and Disease Model Technologies (hDMT)
Initiated in 2015, hDMT is a pre-competitive, not-for-profit technological R&D institute integrating human stem cell technologies with academic and industry expertise to develop human organ and disease models-on-a-chip. Focus areas include vessels-on-chip/vascular disease, heart-on-chip/cardiac diseases, cancer-on-chip and brain-on-chip.
ORgan-on-Chip In Development (ORCHID)
A Horizon 2020 funded EU initiative started in 2017 with the aim of creating an OoC roadmap and building a network of academic, research, industrial and regulatory institutions to move the technologies from the laboratory towards adoption.
The project has finished and the roadmap has been published, outlined in Mastrangeli et al. (2019). The roadmap will continue to be implemented through the European Organ-on-Chip Society established in 2018 as one of the outputs from ORCHID. The Society, a not-for-profit organisation, provides opportunities to share knowledge and expertise in the field, and runs an annual conference.
The Centre for Alternatives to Animal Testing (CAAT)/CAAT Europe T4 Think Tank on MPS
In 2015, a CAAT Transatlantic Think Tank of Toxicology (t4) workshop, brought together global stakeholders to discuss the status of OoC technologies for industry needs and requirements to facilitate adoption and regulatory acceptance. In 2019, a subsequent workshop was held to discuss the barriers that need to be overcome for the technology to be adopted by the pharmaceutical industry and to achieve regulatory acceptance.
- Summary of the 2015 workshop: Marx et al. (2016)
- Summary of the recommendations from the 2019 workshop highlighting where the technology has started to be implemented in the pharmaceutical industry and a roadmap to adoption and regulatory acceptance: Marx et al. (2020)
The UK Organ-on-a-chip Technologies Network
A network funded by the MRC, EPSRC and BBSRC from 2018 to 2022 to capture, inspire and grow the UK research activity in the OoC field. Funding has now ended but the network continues to operate as a forum for members to collaborate, share information, showcase their research activity and support one another. Many universities have now established research centres in this field, such as the Centre for Predictive in vitro Models at Queen Mary University of London. The achievements of the network and the activities undertaken can be found on the website.
The Japanese Agency for Medical Research and Development
The project aims to develop OoCs populated with cells derived from induced pluripotent stem cells that can be applied for safety and pharmacokinetics in drug discovery, with a focus on liver, intestine, kidney and the blood-brain barrier. Summary of the research supported by this project: Ishida (2021).
References
- Edington CD et al. (2018). Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Scientific Reports 8: 4530. doi: 10.1038/s41598-018-22749-0.
- Fabre K et al. (2020). Introduction to a Manuscript Series on the Characterization and Use of Microphysiological Systems (MPS) in Pharmaceutical Safety and ADME Applications. Lab on a Chip 20(6): 1049–1057. doi: 10.1039/c9lc01168d
- Herland A et al. (2020). Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nature Biomedical Engineering. doi: 10.1038/s41551-019-0498-9
- Irrechukwu O et al (2023). Applications of microphysiological systems to disease models in the biopharmaceutical industry: Opportunities and challenges. ALTEX Alternatives to animal experimentation. doi: 10.14573/altex.2204071
- Ishida S (2021). Research and Development of Microphysiological Systems in Japan Supported by the AMED-MPS Project. Front Toxicol. doi: 10.3389/ftox.2021.657765.
- Low LA and Tagle DA (2017a). Organs-on-chips: Progress, challenges, and future directions. Experimental Biology and Medicine 242(16): 1573–1578. doi: 10.1177/1535370217700523
- Low LA and Tagle DA (2017b). Microphysiological Systems (“Organs-on-Chips”) for Drug Efficacy and Toxicity Testing. Clinical and Translational Science 10(4): 237–239. doi: 10.1111/cts.12444
- Low LA and Tagle DA (2017c). Tissue Chips - innovative tools for drug development and disease modelling. Lab Chip 17(18): 3026–3036. doi: 10.1039/c7lc00462a
- Marx U et al. (2016). Biology-inspired Microphysiological System Approaches to Solve the Prediction Dilemma of Substance Testing. ALTEX- Alternatives to animal experimentation 33(3): 272–321. doi: 10.14573/altex.1603161
- Marx U et al. (2020). Biology-inspired microphysiological system to advance medicines for patient benefit and animal welfare. ALTEX- Alternatives to animal experimentation 37(3): 365–394. doi: 10.14573/altex.2001241
- Mastrangeli M et al. (2019). Building blocks for a European Organ-on-Chip roadmap. ALTEX- Alternatives to animal experimentation 36(3): 481–492. doi: 10.14573/altex.1905221
- Maschmeyer I et al. (2015). A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab chip 15(12): 2688–2699. doi: 10.1039/c5lc00392j
- Novak R et al. (2020). Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nature Biomedical Engineering. doi: 10.1038/s41551-019-0497-x
- Tagle DA (2019). The NIH microphysiological systems program: developing in vitro tools for safety and efficacy in drug development. Current Opinion in Pharmacology 48: 146–154. doi: 10.1016/j.coph.2019.09.007
- Wagner I et al. (2013). A dynamic multi-organ chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 13(18): 3538–3547. doi: 10.1039/c3lc50234a
A showcase of organ-on-a-chip research funded by the NC3Rs highlighting the technology's scientific and 3Rs benefits. Recorded in 2020.