The Experimental Design Assistant: From research planning to training tool

A national training initiative in France is using the EDA to embed robust experimental design into real-world research practice.
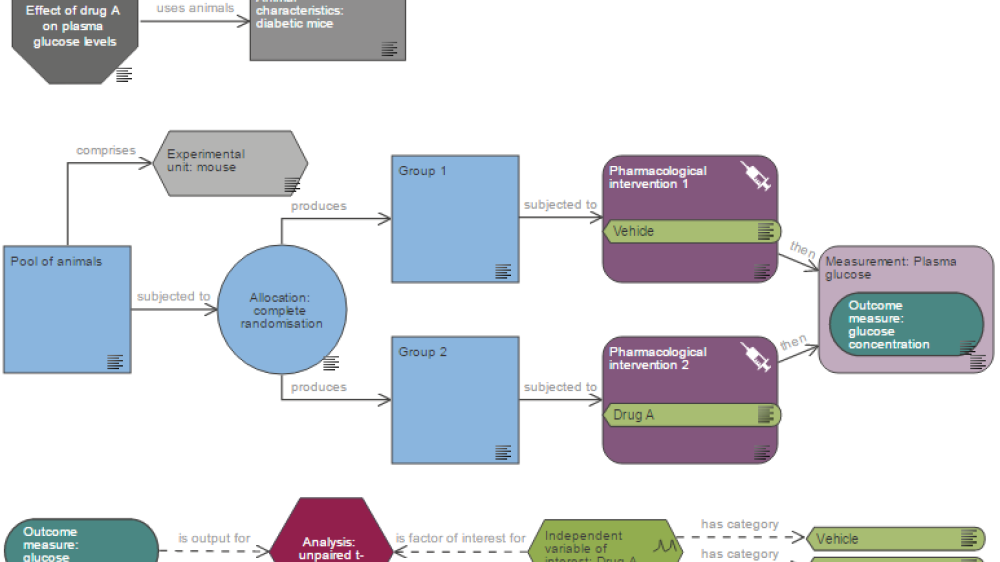
Robust experimental design is a cornerstone of high quality, reliable biomedical research. It is particularly important to ensure that animals are not wasted in poorly designed in vivo experiments that do not meaningfully add to the scientific knowledge base. The NC3Rs Experimental Design Assistant (EDA) is an online tool which guides researchers in designing their experiments. Once a user maps out their experimental plan the EDA provides feedback to improve experimental design and ensure plans use the optimal number of animals needed to give reliable results and answer the scientific question. The EDA currently has over 22,000 users and provides researchers with a wealth of experimental design information and guidance alongside the tool. As well as supporting researchers to design their own experiments, the EDA can be used as a training aid to teach experimental design and share best practice.
Inserm*, the French National Institute of Health and Medical Research, has developed an experimental design training course called Parcours-EDA. Following on from a train-the-trainer session delivered by Dr Esther Pearl (Programme Manager – Experimental Design), Inserm members formed a training network using the EDA to teach experimental design for in vivo studies across research institutes in France.
Esther caught up with Dr Brigitte Rault, the organiser of Parcours-EDA, to find out how they set it up and any advice they have for others interested in using the EDA in teaching and training.
What is Parcours-EDA?
Parcours-EDA is a training course designed to provide those working with animals with concrete ways to improve the scientific and ethical quality of their projects.
We wanted to not only inform researchers in our laboratories about the EDA, but encourage them to use it by providing support for improving real projects.
The course is made up of several parts and training formats: an introductory webinar, video capsules that cover certain basic statistical concepts needed to use the tool optimally and practical workshops in small groups led by experienced trainers. Each part of the course gives rise to a certificate of completion, which can be counted towards the statutory training hours required to maintain skills in France.
Can you explain the three parts of the training pathway?
- A 3.5-hour introductory webinar covering the concepts of experimental bias and statistical testing, with a live demonstration of the EDA. The webinar recalls the essential points in the construction of a robust experimental plan and presents the EDA as a tool to support this.
- A series of 10-minute videos on basic statistical concepts (randomisation, block design, etc.) freely available on our website. There is a knowledge-check quiz after each video to help consolidate learning.
- A practical workshop (4 hours) on the use of EDA in small groups, for those that have followed the webinar and passed the quiz on all the video capsules. We felt that this workshop was essential because the EDA is a powerful, comprehensive tool, but some users need support to get the most out of the platform. These workshops really help to motivate researchers, as they are supported in using the tool and discovering its features.
Who is the course for?
The video capsules are freely accessible online for all researchers. The webinar and small group workshops are open to Inserm researchers or researchers working in an Inserm laboratory and developing scientific projects using an animal model. Before attending a workshop, researchers must have previously completed an approved training course for project designers (in the UK this would be researchers who hold a Personal Licence). In the future we may open Parcours-EDA to members of ethics committees.
Tell us more about the network of trainers who run Parcours-EDA
The practical workshops are delivered by a network of trainers, who also recruit new trainers and experimental design consultants to support the course.
This network approach to delivering training has allowed us to expand the reach of Parcours-EDA and train people at more institutions. We started with a group of 12 trainers and now have a total of 32 trainers from 13 institutions across France.
After completing the course, anyone can become an experienced trainer by co-facilitating at least two workshops. The idea is that those that take the training will spread good practices and raise awareness among their colleagues, either by alerting them to the risks of experimental bias, motivating them to follow the course or by joining the network and becoming trainers themselves.
What are the benefits of using the EDA in teaching and training?
The EDA provides an overview of all the key steps and questions involved in designing a robust project using animals. It provides essential methodological support in this process and helps to raise researchers' awareness of the importance of the conditions under which studies are carried out. In the workshops we offer trainees the opportunity to gradually acquire confidence and independence to construct EDA diagrams based on a bank of ten projects of increasing complexity, devised by the group of trainers. This approach allows them to fully understand the concepts of factorial design, block design, nuisance factors, nested variables and cross-over design and to see scenarios that may correspond to the profile of their project.
Course participants appreciate the questions that EDA prompts them to ask. They also appreciate the graphical representation, which makes it easier to understand the different stages of the project. The youngest students are very familiar with IT tools and quickly grasp the functionalities of the software.
How many researchers have you delivered training to so far?
We launched Parcours-EDA in 2024 with three sets of webinars, which included live use of the EDA attended by over 800 participants. The coaching workshops from the NC3Rs on learning and using the EDA enabled us to provide in-person, interactive training for over 200 people throughout France. We are really pleased that the statistics video clips are the most consulted course on Inserm's e-learning platform in 2024, with more than 500 users. Our latest EDA webinar, held in October 2025, was attended by 200 people.
In total, so far we have reached approaching 1,000 researchers.
Do you have any advice for organisations who want to set up a similar course?
Our system is based on a dynamic network of trainers. The choice of members of the initial group is therefore important. It is preferable to recruit people who are well integrated into the research ecosystem (members of platforms, members of ethics committees, etc.) and have experience in project design or teaching skills.
Trainers should not be presented and considered as experts, but rather as coaches. Most important is that they are motivated and friendly!
To ensure the sustainability and development of this type of programme, it is important to be able to recruit new trainers and support them in joining the network. I would recommend the joint production of a single set of support documents, as it keeps the training consistent across all geographical locations, makes it easier to replace trainers, ensures that everyone understands the content and, of course, makes sure that the work is shared!
When we initially launched we held webinars which included live use of the EDA by all participants. With up to 300 people in each webinar, it was difficult to respond to all the requests for technical or functional assistance simultaneously! For this reason, going forward we opted to include only a demonstration of the EDA by the trainer in the webinar and then provide bespoke support during the small group workshops.
Inserm and the 3Rs
Inserm is a public scientific and technological institute dedicated to biomedical research and human health. It is concerned about the application of good ethical practice in clinical and preclinical research. Supporting researchers to put the 3Rs into practice and improving experimental design in animal research is part of their work to improve reproducibility. Inserm played a leading role in the creation of the French 3Rs Center (FC3R) which it currently chairs.
Useful resources
- If you are new to the EDA, find advice on how to get started on our user support pages.
- For anyone involved in designing experiments or teaching experimental design we hold regular virtual EDA workshops that include time to use the EDA with expert help available.
- The EDA website has a library of information pages on experimental design, from the fundamentals of hypotheses, variables and outcome measures, to how to account for animal characteristics, implement randomisation and blinding and choose the appropriate statistical analysis for an experiment.
- Read our EDA tips article to find out more about the features, tools and support available and make sure you are getting the most out of the EDA.
- Experimental design and transparent reporting are two sides of the coin for reproducible in vivo research that uses the fewest number of animals to generate reliable results. Explore our ARRIVE training resources for support to understand, assess and implement best practice in the reporting of animal research.
EDA training
If you are interested in a train-the-trainers course on experimental design and the EDA at your institution please get in touch via eda@nc3rs.org.uk.
If you are based in France at an Inserm institution register for the Inserm EDA webinar and find a Parcours-EDA trainer in your region:
| Parcours-EDA trainer | Institution |
|---|---|
| ARABO Arnaud | Université (Rouen) |
| AUBAILLY Nathalie | INSERM (Bordeaux) |
| BARROT Laura | INSERM (Lyon) |
| BESSERER-OFFROY Elie | Université (Caen) |
| BONNEFONT Anne-Laure | INSERM (Montpellier) |
| BOURREAU Jennifer | INSERM (Angers) |
| DANGLES-MARIE Virginie | Institut Curie, Faculté de Pharmacie (Paris) |
| DRUILHE Anne | INSERM (Limoges) |
| DUPRAT Fabrice | INSERM (Nice) |
| GERVASONI Damien | CNRS (Lyon) |
| EL OUSSINI Hajer | CNRS (Bordeaux) |
| JACQUOT Fréderic | INSERM (Nantes) |
| JOURNIAC Nathalie | Université (Lille) |
| LANGONNET Stéphane | Centre Léon Berard (Lyon) |
| LE Bon Agnès | INSERM (Paris) |
| LORIVEL Thomas | CNRS (Nice) |
| LUCAS Anthony | INSERM (Grenoble) |
| MALLEM Yassine | ONIRIS (Nantes) |
| MARTINEZ-CORRAL Inès | INSERM (Lille) |
| MELLO Marielle | INSERM (Marseille) |
| MERCIER Emeline | Université (Grenoble) |
| OBINO Dorian | INSERM (Paris) |
| POUSSIER Sylvain | Université (Nancy) |
| RAULT Brigitte | INSERM (Paris) |
| REMY Gaëlle | CNRS (Lille) |
| ROUAHI Myriam | Université (Toulouse) |
| SANSONI Amandine | INSERM (Marseille) |
| SCHROEDER Henri | Université (Nancy) |
| SOULAGE Christophe | Université (Lyon) |
| THOMAS Lionel | CNRS (Strasbourg) |
| VAN ES Armelle | INSERM (Bordeaux) |
| WALIGORA-DUPRIET Anne-Judith | Université Paris Cité (Paris) |
Read our interview with lecturers at the University of Nottingham who have incorporated the EDA into undergraduate teaching.

Regular online workshops featuring a live demonstration and personalised feedback on your experimental design.