Blood sampling: Vascular catheters
Catherisation in laboratory animals can be considered for long-term sampling or multiple sampling over a relatively short time period.
On this page
Introduction
Catheterisation is not only useful for blood sampling; it can also be considered for repeated substance administration or continuous ambulatory infusion. Catheters can be used to access deep, normally inaccessible vessels (e.g. the hepatic portal vein) and temporary cannulas can also be considered for superficial veins. Technical developments have made automated blood sampling possible in animals with implanted catheters.
The ethical review of research protocols proposing to use implanted catheters should carefully weigh the benefits against the costs of the surgical procedures required to implant catheters (e.g. potential pain, discomfort and distress) and the risk of long term complications, such as infection, thrombosis (which dependent on the site can result in stroke) and reactions to implant materials.
The main challenges to successful long-term vascular access are:
- Preventing thrombosis, which can lead to catheter and vessel blockage (occlusion).
- Using appropriate catheter material, design and construction.
- Preventing catheter-related infection.
- Restricting animal interaction with the catheter (only applicable for externalised catheters; animals can be group housed when implanted with transcutaneous buttons).
Good planning, experimental design, aseptic technique and surgical technique are essential to ensure optimum research outcomes from animals with vascular catheters.
Vascular access ports
As exit sites of exterorised catheters pose a major risk of catheter infection, totally-implanted systems using vascular access ports (VAPs) were developed and are used on large animal species, such as dogs, pigs and non-human primates; however, they are not ideal for rodent species due to the need to access the port through the skin.
VAPs consist of an implantable chamber attached to the catheter (Figure 1). The chamber has a thick polymer septum which is capable of withstanding repeated punctures, and provided that specially designed (Huber) needles are used, it has a self-sealing action (Figure 2).
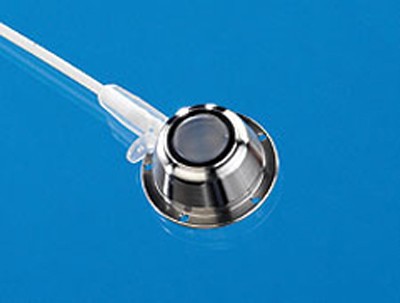
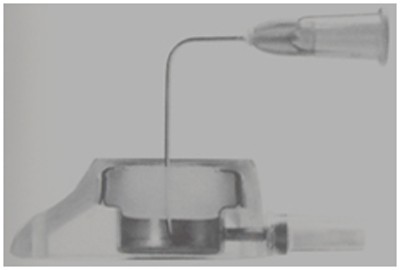
Puncturing the skin overlying the VAP is necessary to access the lumen. Since the septum of a VAP is very thick it gives stable support to the needle and it is possible to leave the needle in situ for many hours or days if required, provided it is suitably protected. Following needle withdrawal, no special care is required.
Transcutaneous skin buttons
Skin buttons are closed systems with exteriorised septums that are implanted subcutaneously and connect to surgically implanted catheters (Figure 3).

Utilisation of skin buttons offers benefits that align with improved animal welfare and scientific outcomes:
- Animals implanted with skin buttons can be group-housed post-operatively (with use of a protective cap).
- When implanted under sterile conditions and accessed aseptically, skin buttons can provide several months of patency.
- Improved patency rates can reduce the number of animals needed for study.
- Animals may be reused on multiple studies when possible.
Potential issues that may be observed with skin button implantation are outlined below:
- Seroma formation post-operatively. Good surgical technique, minimisation of dead space when creating the skin button pocket, and avoidance of anchoring the skin button to underlying muscle/subcutaneous tissue can alleviate this. Seromas are not uncommon to observe post-surgically and should not have an impact on patency or usability of the animal on study.
- Skin abrasions around the skin button due to scratching may indicate post-surgical pain. Ensure an appropriate analgesia protocol has been utilised to mitigate the risk of pain. The animal's nails can be trimmed at the time of surgery when needed.
- Incisional dehiscence. Utilise appropriate suture material for skin closure and ensure the button pocket is the appropriate size.
- Infection of the skin incision or catheter. Ensure aseptic technique has been adhered to at the time of surgery but also anytime the button is accessed.
- Inability to withdraw blood or flush the transcutaneous skin button. Ensure positive pressure technique is utilised when accessing the button and that the catheter is flushed and locked following an appropriate maintenance interval.
Preventing thrombosis
Circulating blood has the capacity to clot (form a thrombus) at sites of injury, foreign materials or where blood flow is either slowed or irregular (due to stasis or turbulence).
A thrombus can arise on the blood vessel wall or on the surface of a catheter and in time, due to cellular factors being deposited, undergo transformation into a fibrin sheath. A thrombus within the vessel lumen around a catheter tip can cause partial blockage permitting infusion of substances while preventing withdrawal of blood. If extensive, the thrombus may cause total blockage (occlusion). Another risk of thrombosis is thromboembolism. Minimising the potential for thrombus formation can be achieved by:
- Selecting materials with low thrombogenicity to minimise thrombus formation on the catheter surface, such as silicone or polyurethane.
- Using atraumatic surgical technique and suitably flexible ('soft') catheters with low surface friction and rounded tips to minimise vessel trauma. Vessel trauma can lead to thrombosis around the implant.
- Surgical staff who have appropriately trained in microsurgical techniques will have a direct impact on prolonged patency in catheterised animals.
- Positioning the catheter tip in a flowing blood stream can help prevent thrombosis due to stasis. Some types of catheter have a tip configuration which acts as a valve preventing ingress of blood and luminal thrombosis (e.g. a Groshong catheter). Ensure the catheter tip is always placed in its optimal location within the vascular system.
- Exteriorise the catheter using a transcutaneous button with a septum that creates a closed system. This permits aseptic technique when accessing the catheter, keeps the catheter subcutaneous to prevent evaporation and subsequent clotting, and allows for group housing when the exterior portion of the button is protected (see below).
- Good catheter use and management will also minimise the possibility of thrombosis. This involves minimising blood residues in the catheter lumen by careful flushing and locking (see below).
- Be aware that thrombosis risk is greater in arterial catheters due to higher pressure having the potential to force blood into the catheter lumen. Use of such catheters requires careful management.
Catheter locking and access
It is essential to employ aseptic technique any time a transcutaneous skin button and catheter are accessed. Strict adherence to aseptic technique will directly impact patency duration and animal welfare.
Catheters that are connected to skin buttons do not need to be maintained more than once weekly. A low-concentration heparin/saline flush solution should be used for catheter maintenance. All solutions used to access the catheter should be sterile and pharmaceutical grade.
During catheter access it is important to minimise the time in which blood remains stationary in the lumen. Immediately after the blood samples have been collected, the catheter lumen should be flushed thoroughly before blood begins to clot. During the time periods when a catheter is not being accessed, the dead space of the catheter should be filled with a suitable sterile high-concentration heparin locking solution to exclude blood and prevent clotting - this process is called locking. Positive pressure technique should be used so that blood is not pulled into the catheter during the process of accessing it.
Locking technique and solutions are also important in preventing infection. A wide range of flush/lock solutions and techniques are used and have been described (also see Table 1 below). The choice of flush/lock solution should be made to ensure that its composition minimises experimental interference (e.g. heparin may be undesirable if blood clotting mechanisms are being studied and taurolidine is an antibacterial compound).
Table 1. Catheter flush and lock solutions
| Flush/lock solution | Advantages | Disadvantages | Notes |
|---|---|---|---|
| 0.9% saline |
|
|
|
| 0.9% saline with heparin (10-20 IU/ml) flush solution |
|
|
|
| 0.9% saline with heparin (100-500 IU/ml) lock solution |
|
|
|
| 40% dextrose (glucose) lock solution |
|
|
|
| 50% sucrose (saturated) |
|
|
|
| Glycerol |
|
| |
| Polyvinylpyrrolidone (PVP) |
|
| |
| Sodium citrate |
|
| |
| Taurolidine citrate solution 6.7% |
|
|
|
Catheter design and selection
The biological, chemical and physical properties which are required for optimal vascular catheter design and construction are listed in detail in the list below. There are many considerations, for example:
- Vascular catheters come in contact with blood and vascular endothelium, as well as other body tissues such as skin and connective tissue. The intravascular tubing material can rapidly become coated in biofilm derived from the animal's circulating blood. The biofilm acts as a substrate for thrombosis and microbial colonisation. The nature of the catheter material and any surface coatings applied influences the quality and quantity of biofilm that forms.
- Catheters should be compatible with the chemical compounds or solvents administered during experiments and must not bind substances of interest during blood withdrawal. Air permeability should also be considered.
- Catheter strength, material, and durability are also important properties.
It is therefore recommended to use commercially purchased, sterile catheters, and to seek advice from suppliers on what type of catheter is more suitable for your specific research needs.
Desirable properties of vascular catheter materials:
Biological
- Non-irritant - provokes minimal inflammatory response
- Non-carcinogenic - low tendency to cause neoplasia
- Non-thrombogenic - low tendency to cause blood clotting
- Non-toxic
- Resists microbial adhesion
- Resists biofilm deposition
Physical
- High tensile strength
- Resists compression - maintains lumen patency
- Optimum flexibility
- Low friction coefficient
- Dimensional stability
- Tolerates physical sterilisation methods (e.g. heat, steam, irradiation)
- Ease of fabrication (e.g. heat forming or welding)
- Non-permeable (water, gases, solvents)
- Radiopacity - ability to image catheter with X-rays
Chemical
- Absence of leachable additives (e.g. catalysts and plasticisers)
- Stable during storage
- Stable on chemical sterilisation
- Stable on implantation (non-biodegradable)
- Permits adhesives in fabrication (possibility of bonding dissimilar materials)
- Accepts surface coatings (e.g. hydrogel, antithrombotic, antibacterial)
- Compatibility with chemical compounds and solvents (absence of absorption and chemical reaction)
- MRI (Magnetic Resonance Imaging) compatible
Given the many biological, chemical and physical properties which are required for optimal vascular catheter design it is perhaps not surprising that only a few naturally occurring and synthetic materials have proved suitable for constructing vascular catheters. In practice, there is no single material that can be used for all applications and therefore catheter materials need to be selected based on an assessment of the intended application. For example:
- Flexible catheters can reduce endothelial injury which can lead to thrombosis, but they may be more difficult to insert and advance in the vessel.
- Surface coatings modify catheter properties such as thrombogenicity, friction coefficient or antimicrobial properties, but in experimental surgery it must be remembered that coatings applied to implants may be biologically active and capable of influencing data. Pilot studies may be required to generate data characterising changes caused by such materials and consideration should be given to suitable controls in experiments.
- The physical shape of a catheter tip can play a significant role in reducing endothelial trauma. Many commercially available catheters have a rounded tip (Figure 4) which is considered to be less traumatic than square cut tubing or bevel ended tubing, although the latter is easiest to insert it should not be used in vascular catheterizations.

Preventing infection
Catheter-related infection is a major risk factor which can compromise vascular catheter studies.
Literature reviews provide only a small number of publications reporting implant-related infections in laboratory animals and how to prevent or manage them. However, there is a vast number of publications concerning human vascular catheter-related infection, and also many reports of animal models of implant infections showing that animals are not resistant to surgical and implant infections. As in vivo experiments become more complex and animals with vascular catheters are kept for much longer periods than in the past (i.e. weeks and months as opposed to hours or days), evidence of infection is likely to increase.
Microbial infections can originate externally, via the catheter lumen at the skin/catheter interface, and internally, via haematogenous spread from distant sites. All surgically-implanted foreign materials can potentially cause infection. Steps that should be taken to reduce infection include:
- Using strict aseptic technique when performing surgery and accessing the catheter.
- Prophylactic antibiotics may help reduce the risk of infection, but should not be used to cover for deficiencies in aseptic technique; it is recommended that veterinary advice be sought before using antibiotics.
- Even where implantation has been performed under strict aseptic conditions, catheter infection from the animal's skin or blood borne from distant sites (e.g. wounds) is possible. A pre-operative health examination should be performed in all animals undergoing any experimental surgery to identify possible infection risks and exclude unhealthy animals from procedures.
Exit sites through skin represent a special challenge as the smooth, non-adherent surfaces of catheter tubing that are desirable for the intravascular portion will create a potential opening where it passes through the skin.
This can be avoided with implantation of a skin button; as the catheter is not exteriorised, but connected to a closed system that is implanted subcutaneously (with exteriorised septum) and under sterile conditions. Care should be exercised when accessing the skin button to minimise the risk of contamination to the catheter and system.
Catheter locking
- Catheter locking has already been mentioned in connection with preventing thrombosis. Positive pressure locking technique and use of pharmaceutical-grade sterile flush and lock solutions are also crucial in minimising infection.
- Regardless of the selected sterile solutions that are used to flush and lock a catheter, strict aseptic technique must always be followed.
- Adding antimicrobial agents to catheter lock solutions is possible but the potential influences of these substances on experimental outcome must be considered. It is inappropriate to use antibiotics reserved for clinical use in catheter locking solutions as this may give rise to bacterial populations with antibiotic resistance.
Experimental design
There are many factors and details that should be addressed to ensure a satisfactory outcome of vascular catheter implantation. It is also necessary to consider the details of specific experiments. The exact nature of implants and their anatomical placement will be influenced by the species used and the purpose of the study; for example, the desired blood sampling route and regimen, the location of infusion, and blood pressure measurement.
It is essential that preliminary in vitro tests are performed to establish that compounds and solvents are compatibile with catheters, including any connecting tubing, infusion pumps and reservoirs.
Particular problems that may be encountered with infusion studies include:
- Absorption of the compound by tubing and other materials.
- Crystallisation of the compound.
- Compounds precipitating out of solution at low flow rates in narrow bore catheters and tubing.
The presence of foreign material within the vascular system and exposure to any eluting coatings from catheters coupled with the compounds used in locking solutions can all give rise to biological or pathological changes that may confound experimental data (e.g. microthrombi may arise and be deposited in the lung during infusion toxicology studies). Access to good background data and well-designed control groups will assist with data interpretation.
Glossary
Ambulatory infusion
- An animal is freely moving without need for a tether to connect with the catheter. This is normally only possible with larger animals that can be fitted with jackets to carry an infusion pump and compound reservoir. Totally implanted pumps can sometimes be used in rodents but have size limitations.
Atraumatic
- Minimal tissue injury is caused during the procedure.
Bicompatibility
- Good acceptance of sterile implants by animal tissues after implantation.
Biofilm
- A coating which develops on implanted materials derived from the animal's own tissue fluids and cells.
Catheter
- Flexible tube inserted into body cavities or organs for medical or experimental procedures.
Dehiscence
- Bursting open or splitting along natural or sutured lines.
Endothelium
- The layer of cells lining blood vessels.
Friction coefficient
- Surface friction influences how easily a catheter can be inserted into a blood vessel and any injury to the vessel lining (endothelium).
Haematogenous spread
- Spread of microbial infection through the blood stream.
Thrombogenic
- Property of causing or promoting blood clotting (thrombosis).
Thrombosis
- The process of blood clotting in which normally fluid blood.
Lumen
- The cavity within a catheter through which blood is withdrawn or compound infused.
Septum
- The thick polymer diaphragm allowing sealing a vascular access.
Transcutaneous button
- An implantable closed system, featuring technology that simply and cleanly connects to a surgically implanted catheter; a syringe can be connected to the skin button for direct blood collection, or a tether can be utilised for continuous access. Animals can be group-housed when implanted with transcutaneous skin buttons.
Vascular access port
- An implantable chamber for intermittent access of catheters by puncture via a self-sealing septum with a special (Huber) needle.
Venepuncture
- Insertion of a hypodermic needle through skin into a vein to withdraw blood samples or administer compounds.
References and resources
- Holmberg A and Pelletier R (2009). Automated blood sampling and the 3Rs.
- Morton DB et al. (2001). Refining procedures for the administration of substances. Laboratory animals 35(1): 1-41. doi: 10.1258/0023677011911345.
- LASA (2010). Guiding principles for preparing for and undertaking aseptic surgery: A report by the LASA Education, Training and Ethics section
- Luo YS et al. (2000). Comparison of catheter lock solutions in rats, 51st Annual Meeting of the American Association of Laboratory Animal Science, San Diego, 6 November.
- Nolan TE and Klein HJ (2002). Methods in vascular infusion biotechnology in research with rodents. Institute for Laboratory Animal Research journal 43(3): 175-82. doi: 10.1093/ilar.43.3.175
- Morton DB (1993). Removal of blood from laboratory mammals and birds. Laboratory Animals 27: 1-22. doi: 10.1258/002367793781082412
- Goossens GA (2015). Flushing and locking of venous catheters: available evidence and evidence deficit. Nursing research and practice 985686. doi: 10.1155/2015/985686
- Cousins TR and O'Donnell JM (2004). Arterial cannulation: a critical review. American Association of Nurse Anesthesiology journal 72(4):267-71. PMID: 15354915
- Colas A and Curtis J (2004). Medical applications of silicones. In: Biomaterials Science: An Introduction to Materials in Medicine. (Eds. Ratner BD, Hoffman AS, Schoen FJ, Lemons JE), pp. 697-707. 2nd Edition. Elsevier Academic Press.
- Passerini L et al. (1992). Biofilms on indwelling vascular catheters. Critical Care Medicine 20(5): 665-73. doi: 10.1097/00003246-199205000-00020
- John SF et al. (1995). Adhesion of staphylococci to polyurethane and hydrogel-coated polyurethane catheters assayed by an improved radiolabelling technique. Journal of Medical Microbiology 43: 133-40. doi: 10.1099/00222615-43-2-133
- Tan RHH et al. (2003). Catheters: a review of the selection, utilisation and complications of catheters for peripheral venous access. Australian veterinary journal 81(3): 136-9. doi: 10.1111/j.1751-0813.2003.tb11074.x
- Chebroux A et al. (2015). Ease of intravenous catheterisation in dogs and cats: a comparative study of two peripheral catheters. The Journal of small animal practice 56(4): 242-6. doi: 10.1111/jsap.12318
- O'Grady NP et al. (2011). Guidelines for the prevention of intravascular catheter-related infections. Clinical Infectious Diseases 52(9): e162-e193. doi: 10.1093/cid/cir257
- Rochford ETJ (2012). Influence of material on the development of device‐associated infections. Clinical Microbiology and Infection 18(12): 1162-7. doi: 10.1111/j.1469-0691.2012.04002.x
- Popp MB and Brennan MF (1981). Long-term vascular access in the rat: importance of asepsis. The American journal of physiology 241(4):H606-12. doi: 10.1152/ajpheart.1981.241.4.H606
- Goldmann DA and Pier GB (1993). Pathogenesis of infections related to intravascular catherisation. Clinical Microbiology Reviews 6(2): 176-192. doi: 10.1128/CMR.6.2.176
- Cooper GL et al. (1988). Possible role of capillary action in pathogensis of experiemetnal catheter-associated dermal tunnel infections. Journal of Clinical Microbiology 26(1): 8-12. doi: 10.1128/jcm.26.1.8-12.1988
- Office of Laboratory Animal Welfare (2021). PHS Policy on humane care and use of laboratory animals.
- National Institutes of Health (2021). Guidelines for the use of non-pharmaceutical grade compounds in laboratory animals.
- AAALAC (2022). Accreditation program (FAQs).
- Instech (2017). Seven things to consider when choosing tubing for research.
- Instech (2017). Permeability of catheter and tubing materials.
