Research round-up: Replacing animals in stroke research

Discover NC3Rs-funded science to replace animals in research to understand and treat stroke.
Contents
Introduction
The NC3Rs has been driving progress to replace the use of animals in stroke research since 2009. Strokes are a type of cerebrovascular disease - the fourth leading cause of death in the UK. During a stroke, blood vessels in the brain become blocked (ischaemic stroke) or rupture (haemorrhagic stroke), leading to a lack of blood supply and oxygen to the brain. Scientists working to improve the treatment of stroke have largely relied on animal models, typically mice, to model the cellular and physiological changes in the arteries, vessels and blood cells in the brain during and after a stroke. From fish embryos to cells and simulations, we are supporting replacement approaches for better science in stroke research.
Zebrafish embryos – a partial replacement
Zebrafish larvae are increasingly being used as a partial replacement* in stroke research and to model other biological and disease processes. According to current scientific thinking, zebrafish embryos less than five days after fertilisation are not capable of experiencing suffering and their use is not regulated under the Animals (Scientific Procedures) Act**. As they have visible nervous, circulatory and organ systems, Zebrafish embryos can replace animals in experiments which require intact physiology and where current in vitro models fail to recapitulate key aspects of biology.
NC3Rs Fellow Dr Siobhan Crilly from the University of Manchester explains the ‘bubblehead’ larval zebrafish model of haemorrhagic stroke in this video, created with the support of an NC3Rs Early Career Engagement award.
Siobhan developed the bubblehead zebrafish model during her NC3Rs-funded PhD Studentship in Professor Stuart Allan’s lab to replace experiments which involve inducing bleeding in the mouse brain to mimic a haemorrhagic stroke. In her Fellowship, Siobhan has applied the replacement model to investigate how the brain regenerates after a stroke and demonstrated its applicability for drug discovery and development.
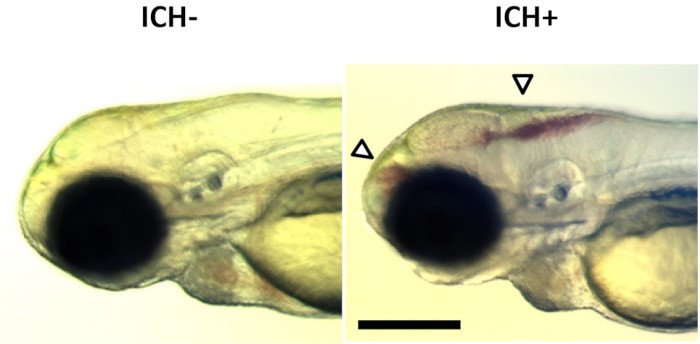
At the University of Sheffield, Professor Paul Evans was awarded an NC3Rs PhD Studentship, co-funded by the British Heart Foundation (BHF), to develop a zebrafish model of atherosclerosis – the thickening or hardening of arteries which can induce ischaemic stroke as well as heart attacks. The bends and branches in arteries alter blood flow, changing the mechanical stress on arterial walls and risk of disease. Research has relied on animal models as this blood flow variation could not be captured in vitro. Paul and his PhD student George Bowley used zebrafish embryos to investigate the molecular mechanisms which lead to atherosclerosis and the genes that determine the risk of stroke and heart disease, without the use of mice.
Human cell-based models of blood clotting
Blood clots, also known as thrombosis, block blood flow and can cause ischaemic strokes and heart attacks. Studies using animal models of thrombosis often have variable results and large numbers of animals are needed to reach a statistically significant conclusion. The NC3Rs and BHF have co-funded a number of projects developing in vitro models of thrombosis, to replace animal models with a more human-relevant alternative.
The rupture of atherosclerotic plaques (a build-up of fat, cholesterol and cells in the arteries) can trigger blood clotting in a process called atherothrombosis. Dr Sarah Jones and PhD student Amelia Drysdale at Manchester Metropolitan University have developed a microfluidic chamber coated with human cells to investigate how the human blood vessel wall impacts atherothrombosis. These microfluidic cell culture devices are known as ‘organ-on-chip’ systems. At the University of Aberdeen, Professor Nicola Mutch, Dr Claire Whyte and PhD student Steven Humphreys are using a ‘thrombus-on-chip’ to investigate how the human body breaks down blood clots. Both groups are aiming to use their models to develop and test new antithrombotic treatments for stroke and cardiovascular disease.
Dr Stephen White worked with Sarah on her project investigating how cellular changes in blood vessels can lead to vascular disease. In his own PhD Studentship, Stephen and student Rhys Smith developed a high-throughput system to screen compounds that could damage endothelial cells – key cells in the blood vessel wall which when damaged can cause blood clots and ischemic blockages. Stephen aims to replace the use of mice fed high-cholesterol diets to assess risk factors for cardiovascular disease and stroke.
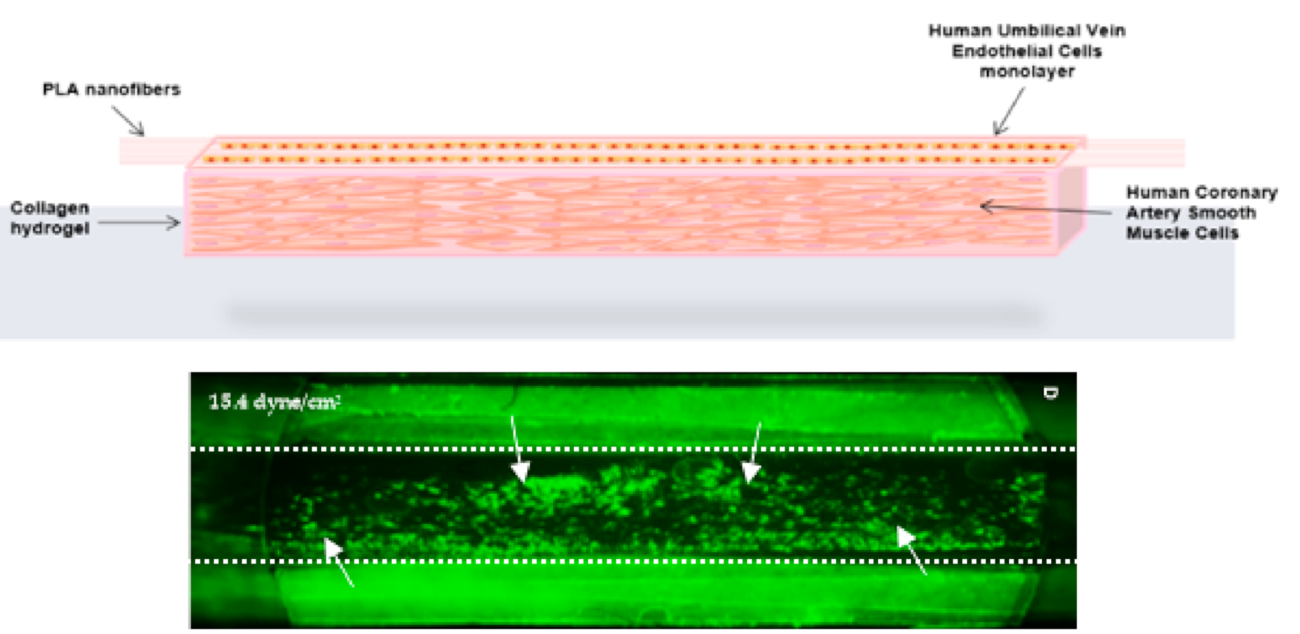
When Jess Berry started her PhD with Dr Matthew Harper at the University of Cambridge, they were intending to use slices of carotid arteries (the main blood vessels that carry blood to the head and neck) collected from mice to investigate thrombosis. By harvesting both carotid arteries from culled mice, this ex vivo model aimed to reduce the number of mice used in vivo by 50% and avoid inducing blood clots in live mice. However, mice lack a key receptor on arterial endothelial cells that is important for thrombosis in humans. Instead, Jess and Matthew decided to replicate human blood vessels in the lab. They perfused channels with human blood, engineering their shape to better replicate the complex network of blood vessels in humans. By inducing blood clotting they were then able to study how thrombi form, travel through arteries and impact the blood vessel network.
Another approach to harness human cell culture to replace the use of animals is tissue engineering. In his PhD research in Dr Alan Harper’s lab at Keele University, Jacob Ranjbar grew human blood vessels in the lab to study how blood platelet cells contribute to blood clotting and vascular diseases. Using human cells and human blood, they were able replace experiments performed in mice. When platelets are ‘activated’ they become sticky, blocking the blood vessel and promoting blood clotting. At sites of injury this is critical to prevent excessive bleeding, but abnormal platelet activation can block the blood supply to the heart and brain.
Dr Alice Pollitt and Professor Jon Gibbins at the University of Reading have developed an innovative method to directly investigate human platelet activation. Typically, research has relied on genetically altered ‘humanised’ mouse models as platelet cells lack a nucleus, making standard molecular biology techniques (such as fluorescent protein markers and genetic knockouts) difficult. Alongside PhD student Carly Kempster, they used ‘fusogenic liposomes’ to carry and deliver compounds into human platelets, building the molecular tools researchers need to replace the use of mice.
Using donated human tissue
Whilst some researchers and growing human tissue in the lab, others are using donated human tissue – the placenta and umbilical cord donated after birth. These tissues have lots of blood vessels allowing researchers to directly investigate bleeding and clotting in real human tissue which would otherwise be discarded.
After developing her 3D human cell model of the arterial wall Dr Sarah Jones is now using donated placentas perfused with human blood. She can use high resolution microscopy to visualise in unprecedented detail how blood clots form in humans. This model replaces experiments which involve amputating the tail of rats to measure bleeding time, which are highly variable and less informative. At the University of Bristol, and with support from the BHF, PhD student Rosaria Bianco and her supervisor Dr Jason Johnson used arterial tissue from human umbilical cords to study how aneurysms form. An aneurysm is a bulge in a blood vessel caused by weakening of the arterial wall. If brain aneurysms leak or rupture the patient suffers a haemorrhagic stroke. Patients are often seen at a later stage of disease than animal models of aneurysm represent, meaning they have limited clinical relevance. Jason estimates their ex vivo model replaced over 700 mice in his lab alone and has the potential to replace around 4,500 mice used in aneurysm research each year.
Stroke research in silico
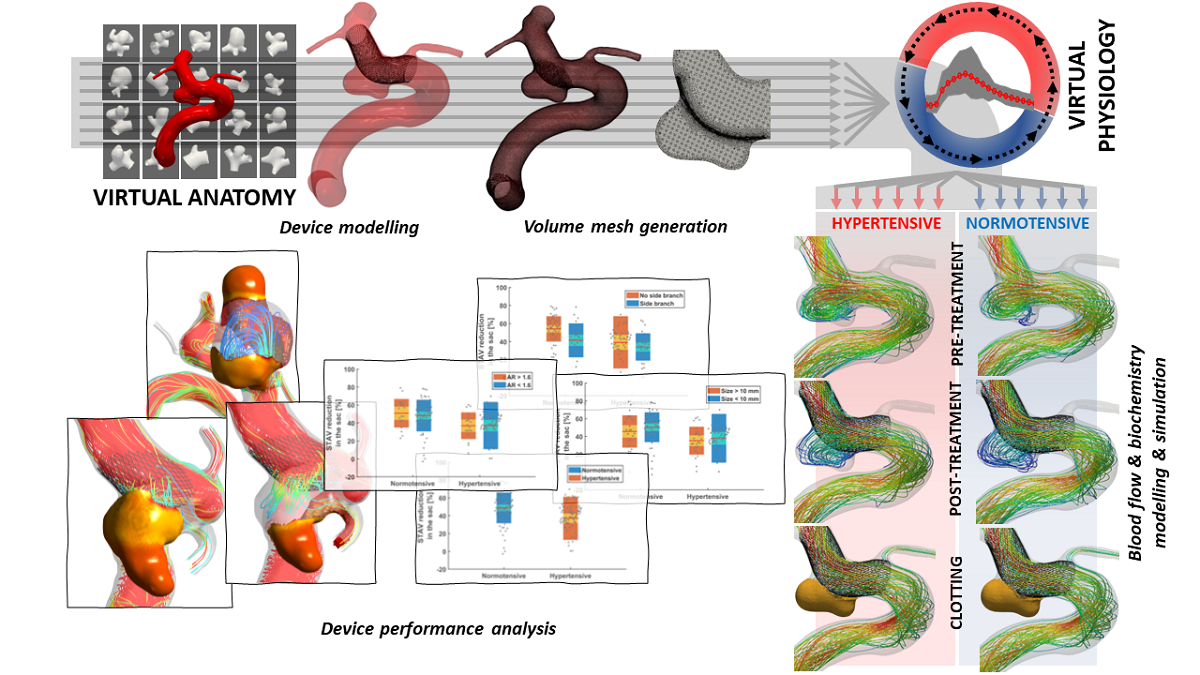
At our annual 3Rs Prize in 2023, the highly commended paper presented an in silico clinical trial for medical devices to treat brain aneurysms. Flow diverting stents aim to re-direct blood flow to reduce the risk of aneurysm rupture. The first-of-its-kind in silico clinical trial on flow-diverting stents replicated findings that took decades and over 4,000 animals, including pigs, dogs and rabbits, to produce in a matter of days. The paper exemplified how computer simulations and mathematical modelling are increasingly able to replace traditional experiments in many areas of research. In fact, Professor Alex Frangi and Dr Ali Sarrami-Foroushani’s virtual trials went beyond what is possible in animal experiments and human clinical trials and identified new risk factors for treatment failure. In silico modelling has the potential to support clinical decisions to be made on a case-by-case basis and reduce the risk of stroke for individual patients.
*Partial replacement includes the use of some animals that, based on current scientific thinking, are not considered capable of experiencing suffering. This includes invertebrates such as Drosophila, nematode worms and social amoebae, and immature forms of vertebrates such as fish.
**The Home Office issues licenses under the Animals (Scientific Procedures) Act 1986 (ASPA) for the use of protected animals in scientific research and testing in the UK. Under the Act, a ‘protected animal’ is 'any living vertebrate, other than man, and any living cephalopod'. Larval forms of fish and amphibians are protected animals under ASPA once they are capable of feeding independently. For zebrafish, this is five days after fertilisation.
Find out about the £3M the NC3Rs has invested to replace, reduce or refine the use of animals in research to better understand and treat Alzheimer’s disease.
Find out about the work we have funded to build the evidence base for refining the use of fish in research.

