Housing and husbandry: Rat
Information to help refine the housing and husbandry of the laboratory rat.
Introduction
Originally domesticated from the wild brown, or Norwegian rat (Rattus norvegicus), laboratory rats are one of the most extensively used rodents in research [1]. While typically docile, laboratory rats retain many of the behaviours of their wild ancestors and will readily present them if given the opportunity [2].
The Laboratory Rat: A Natural History [2] follows the lives of domestic rats after being released in a large outdoor enclosure. After only a few hours, the newly released rats began to use a hopping gait characteristic of their wild cousins and to dig burrows, something they had not been able to do in the laboratory for many generations (see 6:30 in video above).
Behaviour
In the wild, rats are social animals living in extended family groups. Norwegian rats excavate and inhabit an extensive network of tunnels and laboratory rats exhibit analogous burrowing behaviour when provided with suitable substrate [3, 4, 5]. In the wild such burrows would be occupied by a single dominant male, several females and juvenile animals. In the laboratory male rats can be group housed without aggression provided they are placed together before puberty with the most harmonious male groups typically made up of litter mates [6]. Prior to giving birth, female rats will isolate themselves from the rest of the group and build a nest. When nursing their young, female rats can behave aggressively towards other animals and handlers and should be isolated in a nursing cage prior to parturition [7].
Rats are nocturnal animals and are especially active during the early evening and just before sunrise. They engage in play behaviour (e.g. wrestling and chasing) from a young age and these activities are important for establishing social ties, which adult rats will reinforce by grooming. These behaviours are key to maintaining harmonious groups and should not be misconstrued as aggression [8]. Rats also spend periods of time grooming themselves (auto-grooming), an activity that normally forms part of a routine (e.g. following awakening or eating). Rats will also groom themselves as a displacement activity when anxious or threatened, thus protracted or erratic bouts of auto-grooming may be a sign of stress or ill health in an animal and should be investigated [8].
Rats primarily interact with their environment through scent and touch. Pheromones released from scent glands and present in their urine and faeces are used to communicate information such as health, kinship, readiness to breed as well as states of anxiety or distress [9]. When cleaning rat enclosures, care should be taken not to disseminate unfamiliar or conspecific odours as they risk provoking anxiety or aggression. Particular caution should be given when cleaning enclosures with rats during late pregnancy and the first week following birth [10,11]. Rats also communicate through ultrasonic vocalisations with 22kHz sounds being associated with a state of alarm or distress, while 50kHz sounds reflect a positive emotional state [12]. Their vision is relatively poor, however they are highly sensitive to motion and will startle in response to rapid or sudden movements [13].
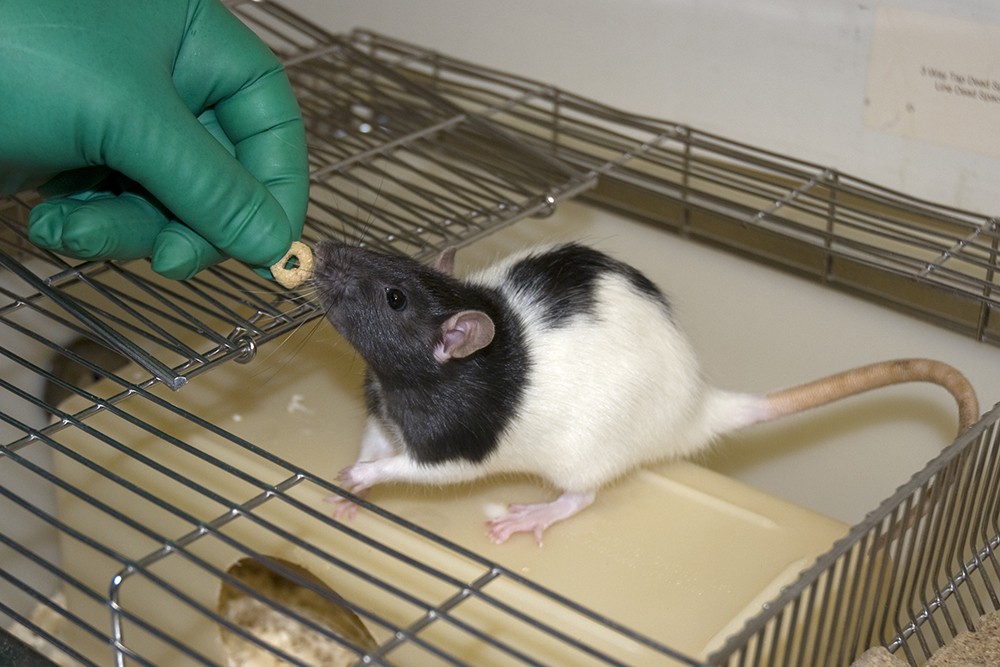
Rats are omnivorous and if handled and housed appropriately they will readily eat most palatable foods offered to them (Figure 1). Stressed or anxious laboratory rats are cautious when exposed to unfamiliar foods making tentative approaches toward and taking small samples from the item until they regard it as safe [14].
Rats are highly intelligent rodents that can be trained to cooperate with handling and procedures, which has a positive impact on both the animals and the handlers. Training techniques for reducing the stress of laboratory rodent were presented at the 2019 NC3Rs/IAT Animal Technicians’ Symposium by Marie Eriksson (RISE).
Housing
Minimum enclosure sizes for rats in European research establishments are fully detailed in Table 1.2 of Annex III to Directive 2010/63/EU. The minimum permitted floor area for group housed adult rats weighing under 600g is 800cm2 increasing to 1,500cm2 for animals over 600g. The minimum permitted height for a rat enclosure is 18cm (Figure 2). Nevertheless, conventional cages might lead to stiffness and positional stress, as they don’t provide enough space for the rats to perform natural behaviours such as stretching and standing upright. Often rats will resolve to lateral stretching in order to alleviate the negative effect conventional cages have on their posture. Therefore, taller enclosures (26-30cm) are strongly recommended to allow the rats to perform their full natural range of movements [5].

Increasingly laboratories are adopting a new type of rat cage, known as the Double Decker, where the total height of the cage is 38cm, providing more space for the rats to perform natural behaviours (Figure 3). The suitability of these Double Decker cages has also been successfully tested in telemetry studies that have historically been performed on animals housed in single-storey enclosures, opening up new opportunities to increase animal welfare during drug safety screening studies.

Rats show a strong preference for enclosures with solid floors, and exhibit signs of stress and discomfort when housed in cages with wire floors without enrichment [15]. This indicates that they should always be housed in solid floored enclosures unless there is a strong scientific justification not to do so.
While typically less aggressive than mice, risk of aggression between rats can be minimised by establishing groups of animals before they reach puberty and providing additional space or isolation for mothers and their litters. Cage cleaning can have a disruptive effect on groups of rats, not solely because it routinely takes place in the daytime when the animals usually sleep, but also due to placing them in a new environment which will result in an altered pheromonal communication [16, 17]. Rat behaviour is affected for roughly an hour post-cleaning, with reports of aggression and even cannibalism in the case of rats close to partition or with new-born pups [11]. Care must be taken to balance the cleaning protocol of rat enclosures with their need to maintain a consistent, controlled environment.
Lighting
Rats are typically housed in artificially lit rooms with a 12-hour light/dark cycle. As a nocturnal species, extended exposure to bright light could have damaging effects to the rat retina, especially albino strains. For that reason, it is recommended that light levels within the enclosure do not exceed 50 lux and racks should ideally have shaded tops to reduce the risk of retinal degeneration [18].
Temperature
UK guidelines recommend that group-housed rats should be kept at a temperature range between 20°C to 24oC [19]. Cages should be provided with a suitable nesting material to allow rats to regulate their microclimate, in addition to allowing them to perform their natural nesting behaviours.
Summary of the key housing features
Rat enclosures must:
- Be large enough to provide enough space for exercise and normal social behaviour.
- Be designed with a minimum height of 18 cm, and preferably 26-30cm or more.
- Be well ventilated.
- Have solid floors covered with an adequate depth of appropriate substrate (such as dust free Aspen wood chip, Megazorb or Alpha-Dri) for hygiene, comfort and to permit natural foraging and digging behaviour.
- Contain sheltered areas for resting, security, and managing social interactions.
- Be softly lit and subject to a light dark cycle.
- Kept at a temperature range of 20-24oC.
Environmental enrichment
Rats’ enclosures should be structured and maintained to accommodate their core needs and behaviours in the wild (i.e. social contact, hiding, exploration, climbing, foraging, gnawing, and resting) [20, 21]. They should be provided with substrate for bedding and insulation at a depth that allows them to engage in digging and foraging behaviour. Studies have shown that size and manipulability are important determinants of bedding preference for rats, with the animals preferring wood-based bedding with a larger particle size such as dust free Aspen wood chip or Megazorb [22]. Female rats close to partition will seek out and build nests and should be provided with a suitable nesting material such as paper strips [23]. Naïve male and female rats will also spontaneously build nests if appropriate nesting material is offered to them [24]. Use of nesting material in rat cages has been shown to have a positive effect on both rat physiology and psychology and should be considered as an enrichment in any rat enclosure [25].
Dependent on the suitability of the resources, rats prefer a source of shelter above other enrichments such as nesting material, though given the option they will choose to have both [23]. As such, a shelter hut or box should be included in every cage. Rats prefer shelters with one entrance rather than open-ended tunnels, and long strips of nesting material rather than short ones [26]. If the nest box provided is not suitable, they will only use nesting material, and vice versa [27]. If space allows, a hammock should also be included in the cage, though it should not substitute for a nest box unless it is fully enclosed, allowing rats to shelter from the light when sleeping.
Like many rodents, rats’ front teeth continue to grow throughout their lifetime. Provision of hard items to gnaw on is essential to prevent overgrowth. A hard-pelleted diet should be considered a minimum requirement to maintain tooth length, while inclusion of wood blocks or chew sticks should also be considered. Note that rats may exhibit individual preferences for which item they chew. Additional nutritional enrichment, such as supplementation with different foods or food textures, should be considered if it does not risk influencing the results of study being performed on the animals.
Rats are social animals valuing contact with other rats over that of an enlarged or enriched enclosure [28]. Removing a rat from a stable social group will result in the rest of its cage mates displaying signs of stress [29]. Rats should not be housed alone without exceptional scientific or animal welfare justification. Where this is necessary, the period of isolation should be kept to a minimum. Rats benefit from being able to make choices about their environment; there is evidence that nursing dams benefit from loft access, which gives them the option to spend away from their pups [30].
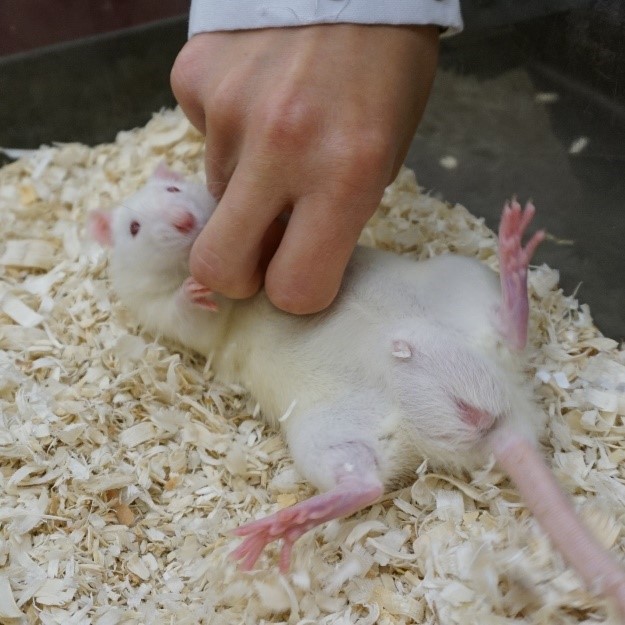
Positive human interactions are also a source of dynamic enrichment for rats. If acclimatised at a young age, they will quickly become receptive to handling (Figure 4).

Tickling or playful handling (Figure 5) as opposed to stroking of young rats has been shown to reduce the fearfulness these animals display towards humans when compared to not handling them at all. It can also decrease the aversion rats display to scientific procedures such as intraperitoneal injections [31, 32]. A program of acclimatisation and handling should be considered a standard component of a rat enrichment programme, especially for those animals that will be used for scientific procedures. More information and resources can be found on our rat tickling pages, produced in collaboration with Dr Megan LaFollette and the Gaskill Lab at Purdue University.
Play and exercise areas can be used to improve rat welfare, improving both their physiological and psychological wellbeing [33, 34]. Playpens for rats (Figure 6) can be easily created by reusing and repurposing materials in the animal facility (e.g. modified rabbit cages), and enriched with water trays, ladders, ropes, tunnels, substrate and treats scattered throughout. Rats can be given regular or intermittent access to these enriched playpens, from a few minutes to a few hours a day, where they can socialise, exercise and perform natural behaviours that cannot be easily performed in their conventional home cages, such as foraging, climbing, and burrowing. Rats are typically exploratory and receptive to novelty [35]. For more information on the benefits of playpens and practical advice on how to set up your own playpen for rats, see the rat playpen resource.

Summary of environmental enrichment options
- Rats should be socially housed and kept either in single sex groups or in stable breeding pairs or trios.
- Rats require objects to gnaw on (e.g. soft wood blocks, hard pellets, cardboard tubes) to prevent their teeth overgrowing.
- Rats should be provided with nesting material from a young age (e.g. paper strips, sizzle nest, nestlets) to enable nesting behaviour.
- You should consider providing rats with access to playpens where they can exercise and socialise.
- At least one shelter (e.g. cardboard tube or plastic house) should be provided to encourage exploration and provide refuge.
- A programme of acclimatisation and handling will improve the rat-handler relationship and reduce the stress of scientific procedures.
Related resources
-
Makowska IJ (2020). The Rat. In: Animal-centric Care and Management, 1st edition. CRC Press.
-
Pritchett-Corning K (2015). Rats. In: Comfortable Quarters for Laboratory Animals (Eds. Liss C, Litwak K, Tilford D, Reinhardt V), Animal Welfare Institute.
-
NC3Rs/AstraZeneca (2017). IAT Congress 2017 workshop summary: Playtime for Rats.
-
Koolas J (2010). The Rat. In: The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals (Ed. Kirkwood RHJ), Wiley-Blackwell.
-
Berdoy M (2002). The Laboratory Rat: A Natural History. Film (27 mins), available at www.ratlife.org.
References
- Home Office (2019). Annual Statistics of Scientific Procedures on Living Animals Great Britain 2018, APS Group.
- Berdoy M (2002). The Laboratory Rat: A Natural History. Film (27 mins), available at www.ratlife.org.
- Boice R (1977). Burrows of wild and albino rats: effects of domestication, outdoor raising, age, experience, and maternal state. Journal of Comparative and Physiological Psychology 91(3): 649-661. doi: 10.1037/h0077338
- Calhoun JB (1963). Ecology and sociology of the Norway rat. Bethesda, U.S. Department of Health, Education, and Welfare, Public Health Service.
- Makowska IJ and Weary DM (2016). The importance of burrowing, climbing and standing upright for laboratory rats. Royal Society Open Science 3(6): 160136. doi: 10.1098/rsos.160136
- Hurst JL et al. (1999). Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Animal Behaviour 58(3): 563-586. doi: 10.1006/anbe.1999.1165
- Pritchett-Corning K (2015). Rats. In: Comfortable Quarters for Laboratory Animals (Eds. Liss C, Litwak K, Tilford D, Reinhardt V), Animal Welfare Institute.
- Komorowska J and Pellis SM (2004). Regulatory mechanisms underlying novelty-induced grooming in the laboratory rat. Behavioural Processes. 67(2): 287-293. doi: 10.1016/j.beproc.2004.05.001
- Inagaki H et al. (2014). Identification of a pheromone that increases anxiety in rats. Proceedings of the National Academy of Sciences 111(52): 18751-18756. doi: 10.1073/pnas.1414710112
- Burn CC (2008). What is it like to be a rat? Rat sensory perception and its implications for experimental design and rat welfare. Applied Animal Behaviour Science 112(1-2): 1-32. doi: 10.1016/j.applanim.2008.02.007
- Burn CC and Mason GJ (2008). Effects of cage-cleaning frequency on laboratory rat reproduction, cannibalism, and welfare. Applied Animal Behaviour Science 114(1-2): 235-247. doi: 10.1016/j.applanim.2008.02.005
- Panksepp J and Burgdorf J (2000). 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behavioural Brain Research 115(1): 25-38. doi: 10.1016/s0166-4328(00)00238-2
- Douglas RM et al. (2006). Perception of visual motion coherence by rats and mice. Vision Research 46(18): 2842-2847. doi: 10.1016/j.visres.2006.02.025
- Modlinska K et al. (2015). Food neophobia in wild and laboratory rats (multi-strain comparison). Behavioural Processes 113: 41-50. doi: 10.1016/j.beproc.2014.12.005
- Van de Weerd H et al. (1996). Preference for different types of flooring in two rat strains. Applied Animal Behaviour Science 46(3-4): 251-261. doi: 10.1016/0168-1591(95)00654-0
- Abou-Ismail UA et al. (2014). Should cages of laboratory rats be enriched physically or socially. Global Veterinaria 13(4): 570-582. doi:10.5829/idosi.gv.2014.13.04.85237
- Bind RH et al. (2013). The role of pheromonal responses in rodent behavior: future directions for the development of laboratory protocols. Journal of the American Association for Laboratory Animal Science 52(2): 124-129. PMCID: PMC3624779
- Perez J and Perentes E (1994). Light-induced retinopathy in the albino rat in long-term studies: an immunohistochemical and quantitative approach. Experimental and Toxicologic Pathology 46(3): 229-235. doi: 10.1016/S0940-2993(11)80088-6
- Home Office (2014). Mice, rats, gerbils, hamsters and guinea pigs. In: Code of practice for the housing and care of animals bred, supplied or used for scientific purposes, Her Majesty’s Stationery Office.
- Makowska IJ and Weary DM (2019). A good life for laboratory rodents?. ILAR journal 60(3): 373-388. doi: 10.1093/ilar/ilaa001
- Neville V et al. (2022). Thinking outside the lab: Can studies of pet rats inform pet and laboratory rat welfare?. Applied Animal Behaviour Science 246: 105507. doi: 10.1016/j.applanim.2021.105507
- Blom HJ et al. (1996). Preferences of mice and rats for types of bedding material. Laboratory Animals 30(3): 234-244. doi: 10.1258/002367796780684890
- Manser CE et al. (1998). Investigations into the preferences of laboratory rats for nest-boxes and nesting materials. Laboratory Animals 32(1): 23-35. doi: 10.1258/002367798780559365
- Jegstrup IM et al. (2005). Nest-building behaviour in male rats from three inbred strains: BN/HsdCpb, BDIX/OrIIco and LEW/Mol. Animal Welfare 14(2): 149-156.
- Vitalo AG et al. (2012). Environmental enrichment with nesting material accelerates wound healing in isolation-reared rats. Behavioural Brain Research 226(2): 606-612. doi: 10.1016/j.bbr.2011.09.038
- Patterson-Kane EG EG et al. (2001). The cage preferences of laboratory rats. Laboratory Animals 35(1): 74-79. doi: 10.1258/0023677011911390
- Bradshaw AL and Poling A (1991). Choice by rats for enriched versus standard home cages: plastic pipes, wood platforms, wood chips, and paper towels as enrichment items. Journal of the Experimental Analysis of Behavior 55(2): 245-250. doi: 10.1901/jeab.1991.55-245
- Patterson-Kane EG et al. (2002). Rats demand social contact. Animal Welfare 11(3): 327-332.
- Burman O et al. (2008). Removing individual rats affects indicators of welfare in the remaining group members. Physiology & Behavior 93(1-2): 89-96. doi: 10.1016/j.physbeh.2007.08.001
- Ratuski and Weary DM (2021). A break from the pups: The effects of loft access on the welfare of lactating laboratory rats. PloS one 16(6): e0253020. doi: 10.1371/journal.pone.0253020
- Cloutier S et al. (2014). The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. Journal of the American Association for Laboratory Animal Science 53(2): 168-173. PMCID: PMC3966273
- Cloutier S et al. (2018). Tickling, a technique for inducing positive affect when handling rats. Journal of Visualized Experiments 135: 57190. doi: 10.3791/57190
- Spangenberg EM et al. (2005). Housing-related activity in rats: effects on body weight, urinary corticosterone levels, muscle properties and performance. Laboratory animals 39(1): 45-57. doi: 10.1258/0023677052886457
- Hinchcliffe JK et al. (2022) The use of ball pits and playpens in laboratory Lister Hooded male rats induces ultrasonic vocalisations indicating a more positive affective state and can reduce the welfare impacts of aversive procedures. Laboratory Animals. doi: 10.1177/00236772211065920.
- Modlinska K et al. (2019). The impact of changeability of enriched environment on exploration in rats. Behavioural processes 164: 78-85. doi: 10.1016/j.beproc.2019.04.015
