3Rs advice for project licence applicants
Guidance and resources to help researchers address the 3Rs in animal licence applications. Although focused on the UK, the information provided is useful for researchers regardless of their location.
On this page
Introduction
All scientific work carried out in the UK under the Animals (Scientific Procedures) Act 1986 must be part of a programme of work authorised by a project licence. An important part of the ethical review and licensing process is ensuring that the principles of the 3Rs are robustly applied. In order to achieve this, when applying for a project licence, applicants are asked to detail how they have considered and applied the 3Rs to their research. To learn more about the 3Rs and how they can drive better science and animal welfare, view our video presentation.
Below we provide advice and highlight resources to help applicants address the 3Rs aspects of a project licence application. This information is also relevant for more experienced project licence holders and members of the Animal Welfare and Ethical Review Body (AWERB). The guidance was prepared by our Regional Programme Managers who have on-the-ground experience of providing 3Rs advice to applicants.
We focus on each of the ‘Rs’ separately, but first highlight two key resources from the NC3Rs which can be used to identify replacement, reduction and refinement opportunities.
NC3Rs research portfolio
The NC3Rs is the primary funder of 3Rs research in the UK. Head to Our Portfolio to find out more about the research projects we have funded, including new models and technologies that can replace, reduce or refine the use of animals. Use the search box to search for specific terms or apply filters on the project listings page, choosing terms across multiple categories to narrow down the results depending on your model, topic of interest or 'R'.
On each project page you will find a summary of the aims, key impacts and any resulting publications.
NC3Rs gateway
Researchers funded by the NC3Rs are encouraged to publish their 3Rs approaches on the NC3Rs gateway, hosted by F1000Research. Here you’ll find novel 3Rs models and technologies, described in extensive methodological and technical details to allow these methods to be reproduced. To help researchers decide whether the approach could be used in their own research, articles on the gateway include a realistic evaluation of the scientific, 3Rs and practical benefits of the approaches described, along with their current and potential applications, and details of their validation against current “gold standard” models.
Replacement
Replacement refers to the use of technologies or approaches that replace or avoid the use of animals in studies. This includes full replacement which avoids the use of any animals (e.g. human volunteers, computer and mathematical modelling, or established cell lines in place of animals) and partial replacement. Partial replacement generally falls into one of three categories: either the animal species used is not protected by law (e.g. invertebrates such as Drosophila or nematode worms), or is used at a life stage prior to it becoming protected (e.g. mammals, birds and reptiles before the last third of their gestation or incubation period; fish and amphibians before they can feed independently), or cells and tissues are used from animals that have not undergone any procedures other than killing by a permitted ‘Schedule 1’ method. Within the project licence application these full or partial replacements are often referred to as in silico, in vitro and ex vivo methods.
Project licence applicants are asked to detail which non-animal alternatives they have considered for the proposed work and explain why these are not suitable for the research objectives. Including this information in the application helps to demonstrate a thorough understanding of the research field. Applicants should conduct a thorough, well-designed search of the scientific literature using appropriate keywords. A number of searchable databases are available which provide detailed information about alternative approaches and the scientific research they can help support.
Resources on searching for alternatives
In addition to the NC3Rs portfolio and gateway, the following guides and databases provide useful information on alternative approaches:
- The EURL ECVAM Search Guide has been developed to help users find information on alternative strategies and methods to animal-based research.
- Replacing Animal Research has put together a resource on the basic principles of searching for 3Rs information.
- The Animal Welfare Information Centre has tips for searching for alternatives, as well as links to relevant databases.
- The Duke University Library provide advice on how to search for non-animal alternatives and link to a number of freely available databases, most of which are specifically designed to search for alternative approaches.
- NORECOPA has a series of databases, including on alternatives to the use of animals in education and training.
To discover more about the utility of different invertebrate models such as Drosophila visit FlyBase, an online database of Drosophila genes and genomes. Additionally, Wormbase provides information about the genetics and biology of Caenorhabditis elegans and related nematodes. For information on using Galleria mellonella (wax moth larvae) in place of mammals for infection studies visit the Galleria Mellonella Research Centre.
Human tissue
Human cells or tissue can help to replace the use of animal models and may provide more translational testing strategies. Visit our human tissue resource page to understand what types of cells and tissue can be used and where they can be obtained. The UK Clinical Research Consortium has a Tissue Directory with sample collections covering multiple diseases, searchable by age, gender, disease classification, sample type, etc. For those interested in human brain tissue, see information from the UK Brain Banks Network. More information on the regulation of human tissue use can be found in guidance published by the Human Tissue Authority and the MRC.
In vitro / in silico / ex vivo research
Applicants are often using in vitro, in silico and/or ex vivo approaches alongside their in vivo work; or have used these approaches during earlier stages of the research to inform project direction. Relevant examples should be given within the project licence application, including those where animals have been replaced during certain steps (e.g. during early screening) or where ex vivo samples have been obtained following a Schedule 1 method of killing (i.e. animals have not undergone regulated scientific procedures).
More information about in vitro models can be found on the websites of the Institute for In Vitro Sciences,In Vitro Toxicology Society and European Society of Toxicology In Vitro. Further information and guidance about organ-on-a-chip approaches can be found at the Organ on a Chip Technologies Network, while the 3DbioNet is a UKRI-funded network providing information and funding for 3D cell culture models.
For information on moving to replacing animal-derived reagents and products, see our animal-free in vitro technologies resource.
Reduction
Reduction is about minimising the number of animals used per experiment, consistent with the scientific objectives. Well-designed and correctly analysed experiments can lead to a reduction in animal use whilst increasing the robustness and reproducibility of the scientific results. Applicants are given opportunities throughout the project licence application to reassure the AWERB and Home Office that the number of animals used is the minimum number that is consistent with the aims of the project.
The project licence application focuses on the following fundamental aspects of good experimental design: the study design (justification for how experimental and control groups were chosen), avoidance of bias (use of randomisation and blinding) and determining sample size (including justification for the chosen effect size). Here we outline a number of resources in this area and how they can be used in project licence applications to address these fundamental concepts.
Resources for study design
Experimental Design Assistant
The Experimental Design Assistant (EDA) is a free-to-use, online resource provided by the NC3Rs to help scientists design robust experiments more likely to yield reliable and reproducible results. The EDA software is accompanied by experimental design information pages that describe the basic components of the design process, all relevant to the project licence application. These include group and sample size calculation, allocation of experimental units to groups (including randomisation) and blinding.
The EDA software provides tailored feedback on the design of individual experiments, with dedicated support for power calculations, randomisation and blinding. Using an appropriate number of animals and employing a rigorous experimental design ensures that the results are useful and prevents waste. Demonstrating your use of the EDA sends a strong signal to the AWERB that your experimental design has been optimised to yield the best possible data from the lowest number of animals. The automatically generated EDA report can be submitted to the AWERB as part of the review process, and EDA diagrams can be supplied as images within the application itself.
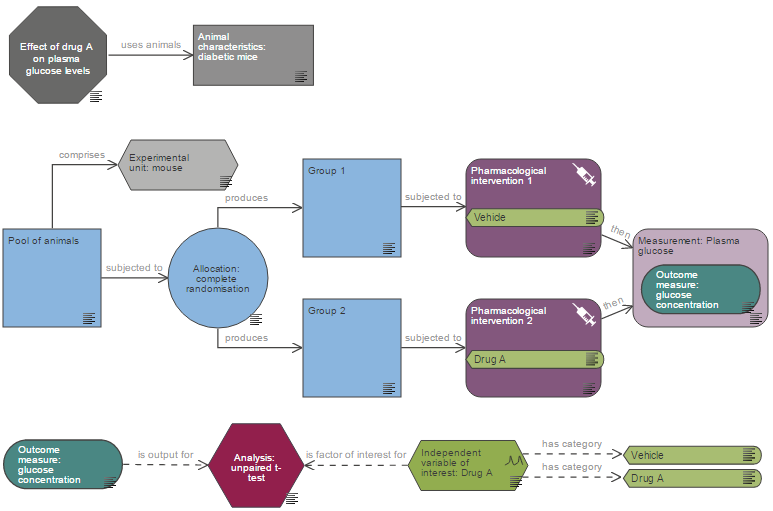
Sample size calculation and justification
Justification for the number of animals required is a critical component of a project licence application. This should be demonstrated by providing the values used in your sample size calculation, and the justification for your chosen effect size (i.e. why this size of effect would be of scientific or clinical interest). The MRC has produced a guidance document with worked examples for justifying experimental design and animal numbers, aimed at grant applicants but equally useful for project licence applicants.
For some experiments, it may not always be possible or appropriate to perform a power calculation. In such cases, we recommend seeking advice from the local statistician. To answer some of the most common questions related to sample size calculations, statistician Dr Simon Bate from GSK has written some helpful guidance on how to decide your sample size when the power calculation is not straightforward.
Reporting and reproducibility
The ARRIVE guidelines were developed by the NC3Rs and experts in the field to improve the quality of reporting of in vivo experiments and the reproducibility of animal research. They have been adopted by over a thousand journals and funders, and most research-intensive universities in the UK – hence many researchers include in their project licence application a commitment to using the ARRIVE guidelines when writing up the results of their research for publication.
The ARRIVE guidelines can also be used as a framework for planning research studies. An Explanation and Elaboration document that accompanies the guidelines provides extensive advice on the design of animal experiments and details the rationale for the fundamental experimental design concepts required in project licence applications.
To minimise unnecessary duplication and ensure all outputs from project licence applications are published, applicants can use the BIH QUEST Fiddle tool (from the QUEST Toolbox for reproducible research) to identify publishing outlets for research outputs that are traditionally more difficult to publish, such as datasets, or negative or neutral results.
General reduction resources
- The choice of sex of animals is an important experimental consideration with potential scientific and reduction impacts. We give advice on how the choice of sex can impact upon your experimental design in the animal characteristics page of the EDA website.
- Pilot studies can be used to gather information to improve the quality and efficiency of subsequent experiments. We have a resource page on conducting a pilot study that provides guidance on how to carefully plan a pilot study, including logistical considerations and what to do with the information obtained.
- Optimising the production, breeding and use of genetically altered (GA) animals is an important means of reduction often overlooked in project licence applications. We have created a resource on breeding and colony management containing examples of efficient breeding strategies and advice on archiving and genetic drift. The resource includes a downloadable guidance document on sharing and archiving of GA mice, which covers resources for finding GA strains to avoid strain duplication, including the Mouse Locator Network and repositories for different species such as the MRC Harwell Archive. It also highlights services for archiving GA lines such as the free MRC Frozen Embryo and Sperm Archive (FESA).
- Organisations such as MRC Harwell and Jackson Laboratories offer resources and courses on efficient breeding strategies for GA mice.
- Experimental design training opportunities include the Replacing Animal Research training schools; local experimental design workshops organised by NC3Rs staff; and use of the EDA software and supporting website.
- We have a series of resources to support reduction strategies in the design of toxicological or regulatory studies, which includes information on efficient study designs in this field, identified by NC3Rs and industry experts, along with a bibliography.
Refinement
Refinement refers to methods which minimise animal suffering and improve welfare. It is the responsibility of the project licence holder to ensure that any regulated procedures specified in the licence cause the least pain, suffering, distress or lasting harm to the animals and are the most likely to provide satisfactory results. Information about refinement is mostly given in the “Refinement” and “Protocols” sections of the licence application.
Sometimes applicants misunderstand the guidance text provided for the Refinement section and repeat information given in the Replacement section, focusing on why non-animal alternatives are not suitable. Instead, you should explain why you have chosen this animal species, model and method to answer your scientific question and why this approach is the most refined for the animals in comparison to other animal-based methods you could have used to address your scientific question.
Applicants may also misunderstand the definition of refinement, confusing it with refinements to optimise their experimental design/data output. Refinements in the context of the project licence application should always refer to those which will benefit the animals and improve their welfare.
Systematic reviews
Systematic reviews can be used to select the most appropriate animal model and determine if less severe procedures are as predictive as more severe alternatives. (They can also be used to inform effect sizes and variability used in sample size calculations). Our webinar on ‘Systematic reviews of animal studies’ provides an introduction to systematic reviews, including the type of research questions that can be addressed, the steps included, tools available and the 3Rs benefits of systematic reviews. One such tool, the Systematic Review Facility (SyRF) is a free-to-use platform funded by the NC3Rs which supports researchers to carry out systematic reviews and meta-analysis of animal studies. It should be noted that systematic reviews can be time-consuming to conduct and typically need to be funded, however they can be enormously useful as part of a wider programme of research. Preparing a project licence (or grant application) provides an opportunity to consider whether a systematic review of the literature would be helpful and what research questions could usefully be addressed using this approach.
General refinement advice
When outlining the refinements to be used, applicants should not forget to include smaller, incremental refinements which impact on animal welfare. These could be refinements to the procedure itself (e.g. choosing the most refined route of administration; habituation to procedures; acclimatising animals to restraint tubes in the home cages) or applying husbandry-based refinements (e.g. providing food on the cage floor for mouse models of arthritis; using non-aversive mouse handling methods).
When suggesting refinements, ensure these have been discussed with the Named Persons, such as the Named Animal Care and Welfare Officer (NACWO) and Named Veterinary Surgeon (NVS), and that they are both relevant to the model and feasible within your facility. Further guidance on planning for experimental procedures is given in NORECOPA’s PREPARE guidelines.
Humane endpoints
A key component of refinement is the implementation of humane endpoints. We have a humane endpoints webpage that provides information on how to establish appropriate humane endpoints that minimise animal suffering. Further advice is given in the Humane Endpoints website of the 3Rs Centre at Utrecht University.
It is important to remember that in many studies pain and distress can be avoided by the identification of non-clinical scientific endpoints that occur before any observable suffering or clinical signs of a condition. Examples of this might include the use of imaging technologies to assess internal tumour burden or the use of study-specific biomarkers in serum. By identifying earlier scientific endpoints this will allow more refined humane endpoints to be implemented, thereby minimising pain and suffering in the models used.
Welfare assessment protocols and score sheets can be useful tools to monitor adverse effects and determine when humane endpoints have been reached. The experience of the Named Persons and animal technicians can be invaluable when creating welfare score sheets that accurately reflect your model by recording relevant adverse effects and incorporating the earliest humane endpoints. The score sheet/s need not be included in the licence application itself but should be shared with the AWERB.
Disease-specific guidance
The NC3Rs has led a series of expert working groups which focus on specific disease models that have the potential to cause severe suffering and opportunities for their refinement. These include:
Use the search box on the Our Portfolio area of our website to identify opportunities to refine the use of other disease models and procedures.
The RSPCA has also produced a series of reports on refining severe models and procedures and has also created a web resource outlining the causes of 'severe' suffering along with practical approaches for reducing or avoiding this.
The Workman et al. (2010) guidelines for the welfare and use of animals in cancer research are an invaluable resource, setting out appropriate humane endpoints and relevant clinical signs to monitor.
Although the examples above are disease-specific guidelines, certain refinements can have wider applications to other models (e.g. social housing post-surgery; wet mash to encourage eating; extra monitoring of animals with co-morbidities).
Refinement resources
The NC3Rs website provides a number of resources to help with further refinement of your research. These include advice and guidance on common procedures such as blood sampling and microsampling, and the evidence base to support the single use of needles as a refinement. We also have species specific pages dedicated to non-aversive mouse handling and genetically altered mice.
Our resource library also contains e-learning resources on conducting welfare assessments and on euthanasia and anaesthesia. Training material on the Procedures with Care resource focuses on the administration of substances to mice and rats and aseptic technique in rodent surgery.
We also have dedicated resources focused on applying the 3Rs in toxicology and regulatory science, with one of the most useful documents being the LASA and NC3Rs guidance on dose level selection for regulatory general toxicology studies for pharmaceuticals.
Staying informed on the latest 3Rs advances
- Project licence holders are ultimately responsible for implementing the 3Rs, and so should have regular discussions with the Named Persons and animal technicians at their institutions to review current approaches and whether there are any new 3Rs opportunities.
- One way to keep up to date with the latest 3Rs developments is to subscribe to the NC3Rs e-newsletter. These monthly updates focus on funding opportunities, 3Rs events and publications.
- For updates on the 3Rs in toxicology and regulatory sciences, subscribe to our quarterly e-newsletter Tox News.
- Attending NC3Rs events and workshops are simple ways of keeping abreast of 3Rs advances and approaches. Check out our events pages regularly to find what’s on offer.
- Registering for our upcoming webinars through our events calendar, or watching the recordings of past webinars.
- Institutional 3Rs symposia are a great way to hear about novel 3Rs approaches, tools and technologies being used by others at your institution.
- Our Regional Team can provide free training, advice and support to facilitate uptake of 3Rs into research practice.
- The NC3Rs has a Technology Partn3Ring platform for small companies and academics to showcase their 3Rs technologies. You can browse showcased technologies and connect with the technology developers on the Technology Partn3Ring innovation platform.
- Other 3Rs organisations produce regular newsletters and host 3Rs events which may provide additional opportunities to keep up to date with the latest advances.
Showcasing our advice and resources to address the 3Rs aspects of licence application forms.

